Question
Question: Acetone + A \( \to \) Oxime of acetone Acetone + B \( \to \) Diacetone amine Acetone + C \( \to ...
Acetone + A → Oxime of acetone
Acetone + B → Diacetone amine
Acetone + C → Mesitylene
Acetone + D → Isopropylidene chloride
A, B, C and D are:
(A) NH2OH,NH3,Conc.H2SO4,SOCl2
(B) HNO2,NH3,Conc.H2SO4,PCl5
(C) HNO2,NH3,Conc.H2SO4,Cl2
(D) NH2OH,NH3,H2SO4,Cl2
Solution
Oxime of acetone contains a carbon-nitrogen double bond. We require a compound which contains nitrogen atoms to get diacetone amine from acetone. Mesitylene is 1,3,5-trimethylbenzene.
Complete step by step solution:
We will find the reagents needed to obtain given compounds from acetone.
- To make oxime of acetone, we know that we require hydroxylamine. Nitrogen atoms of NH2OH attack the carbonyl carbon and then a water molecule is lost to form oxime. The reaction is given as under.
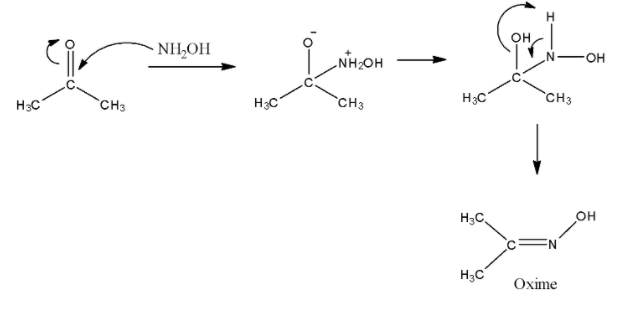
- To make diacetone amine, we require ammonia as a reagent. Actually, two moles of acetone reacts with one mole of ammonia to give one molecule of diacetone amine and water. The reaction requires high temperature. The reaction can be shown as under.
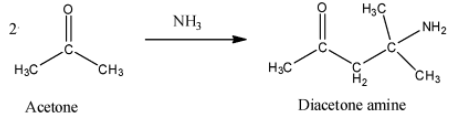
- To obtain mesitylene from acetone, we require concentrated sulphuric acid as a reagent. We require three molecules of acetone to form one molecule of mesitylene. Mesitylene is also called 1,3,5-trimethylbenzene. The reaction can be given as below.
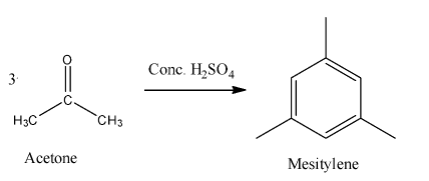
- Isopropylidene chloride is a compound which is a germinal dichloride. That means two chlorine atoms are bonded with the same carbon atom. Thionyl chloride (SOCl2) and Phosphorus pentachloride(PCl5) can convert acetone to isopropylidene chloride. The reaction can be given as
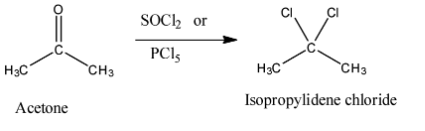
Thus, we can conclude that the correct answer of this question is (A).
Note: Chlorine gas cannot react with carbonyl groups. Actually, chlorine gas in presence of light can do chlorination of alkyl side chains or alkyl groups. Remember that isopropylidene chloride is also called acetone chloride or acetone dichloride as well.
