Question
Question: Acetic acid exists in a dimer state in benzene due to: (A) Condensation reaction (B) Hydrogen bo...
Acetic acid exists in a dimer state in benzene due to:
(A) Condensation reaction
(B) Hydrogen bonding
(C) Presence of carbonyl group
(D) Presence of α-hydrogen
Solution
The dimer of acetic acid has four carbon atoms, four oxygen atoms and eight hydrogen atoms. The reason or force keeping the two molecules of acetic acid together is dipole-dipole attraction.
Complete step by step solution:
We will know why acetic acid exists as a dimer in its solution in benzene.
- Acetic acid has a carbonyl group and one –OH group is directly bonded to the carbonyl carbon. The molecular formula of acetic acid is CH3COOH.
- We know that oxygen atom is an electronegative atom because of its electronic configuration. It just needs two electrons to complete its octet. Hydrogen atoms are electropositive elements.
- The electronegative atoms like oxygen, nitrogen and sulphur can form hydrogen bonds with the hydrogen atoms of the same compound or with the hydrogen atoms of different compounds.
- In the case of acetic acid, the hydrogen atom of –OH group forms hydrogen bonds with the oxygen atom of carbonyl group of other molecules of acetic acid. This results in the formation of a dimer. The structure of a dimer can be given as
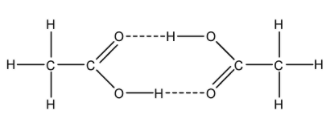
Thus, we can conclude that the dimer is formed because of hydrogen bonding between two acetic acid molecules.
- Condensation reaction is the reaction in which a small molecule is being lost in the reaction between two molecules.
Therefore, the correct answer to the question is (B).
Note: The hydrogen bonds can be intermolecular or intramolecular. Intermolecular hydrogen bonds are the hydrogen bonds which are formed between the atoms of different molecules. Intramolecular hydrogen bonds are the hydrogen bonds which are formed between the atoms of the same molecule.
