Question
Question: Acetate ion contains : a.) One C, O single bond and one C, O double bond b.) Two C, O single bon...
Acetate ion contains :
a.) One C, O single bond and one C, O double bond
b.) Two C, O single bond
c.) Two C, O double bond
d.) None of these
Solution
Hint The ethanoic acid is also known as acetic acid. When a Hydrogen is abstracted from acetic acid, the acetate ion is formed. It is chemically written as - CH3COO−. So, the number of bonds can be seen from the above formula.
Complete answer :
We know that ethanoic acid is also known as acetic acid. When a Hydrogen is abstracted from acetic acid, the acetate ion is formed. It is chemically written as - CH3COO−.
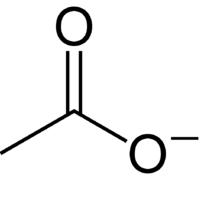
So, it has one C-C single bond, three C-H single bonds, one C-O single bond and one C-O double bond.
So, it contains one C-O single bond and one C-O double bond.
Thus, the option a is the correct answer.
Note : It must be noted that acetate is a monocarboxylic acid anion that formed after removal of a proton from acetic acid. It is a common anion in biology that is used by organisms in the form of acetyl coenzyme A. Acetic acid can undergo a dissmutation reaction to form methane and carbon dioxide. It is an important building block for biosynthesis. These acetic acids combine to give more molecules like fatty acids. The acetic acid is an important chemical used in industries. It is used as a polar protic solvent. It is used as an effective antiseptic.
