Question
Question: Acetanilide can be obtained by the: A. benzoylation of aniline B. alkylation of nitrobenzene ...
Acetanilide can be obtained by the:
A. benzoylation of aniline
B. alkylation of nitrobenzene
C. acetylation of aniline
D. reaction between acetaldehyde and aniline
Solution
We know that, acetanilide is a chemical compound of molecular formula C8H9NO.It exists in solid form in leaf. It is also known by the name acetanil, N-phenylacetamide. By the IUPAC name N-phenylacetamide, we get to know that functional group amide is present in the compound.
Complete step by step answer:
Let’s first discuss the acylation reaction in detail. In this reaction, aromatic and aliphatic primary amines react with acid anhydride, chloride and esters by nucleophilic substitution reaction. In this reaction, a hydrogen atom of amine is replaced by the acyl group and amide is produced. The reaction takes place in presence of a strong base whose basicity is more than amine such as pyridine.
On example of acylation reaction is,
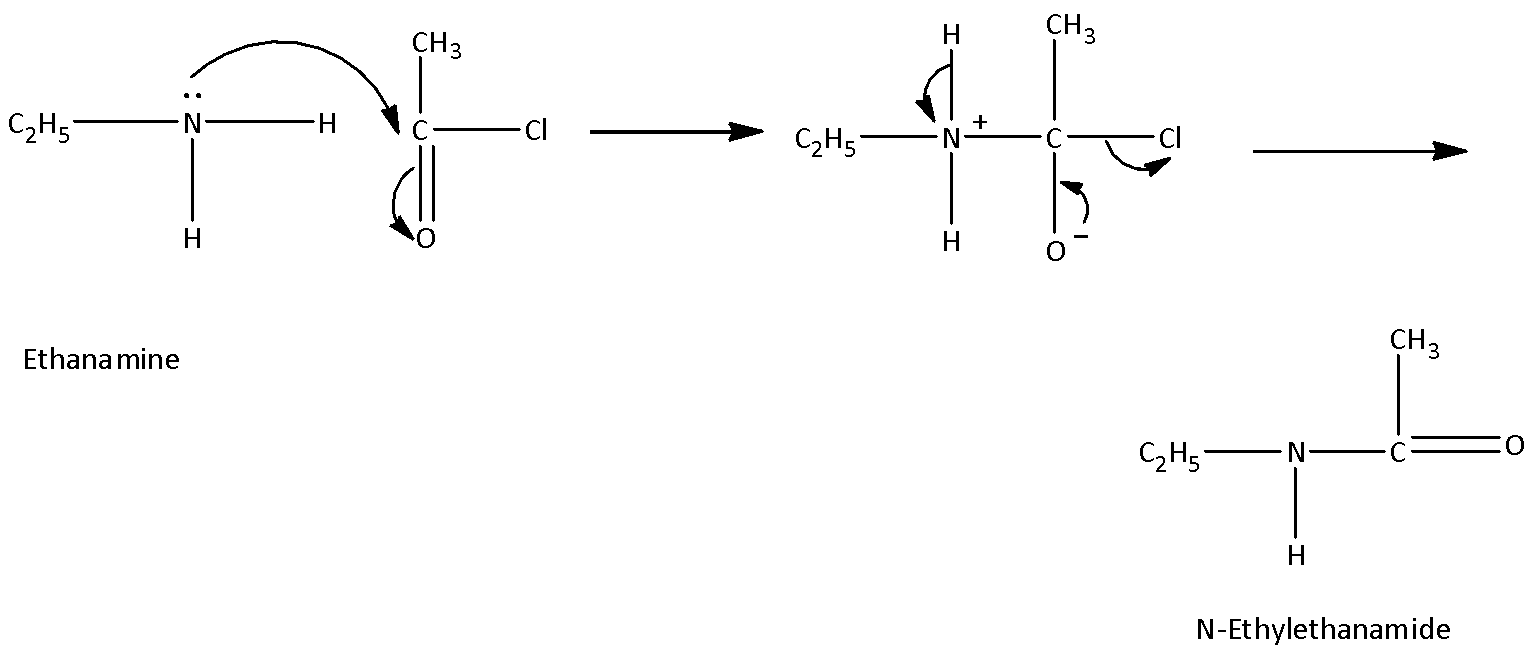
Now, come to the question. The compound acetanilide (N-phenylacetamide) is to be prepared. The product is amide. And one hydrogen atom is to be replaced by acetyl group. So, the reaction by which acetanilide can be formed is by acetylation of aniline.
In the acetylation of amide, aniline reacts with ethanoic anhydride in presence of base like pyridine to form acetanilide.
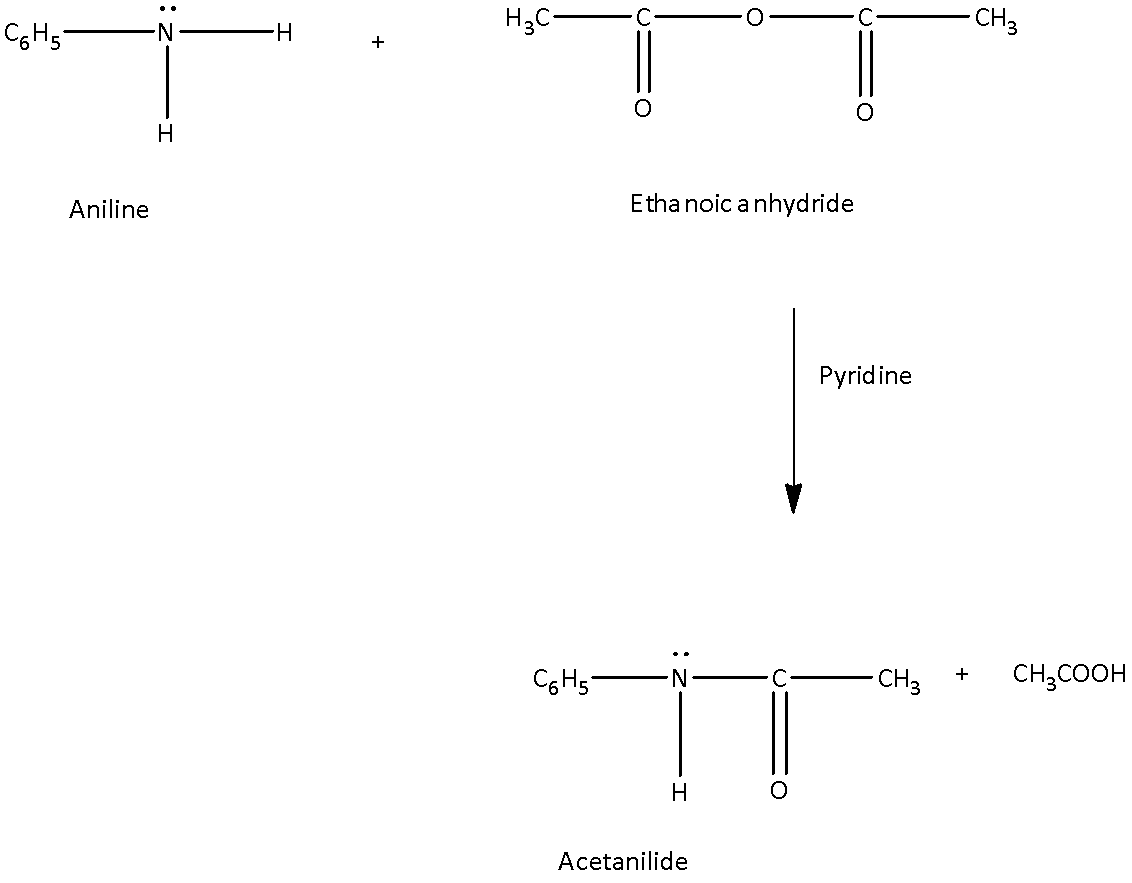
So, acetanilide can be produced by acetylation of aniline.
Hence, the correct answer is option C.
Note:
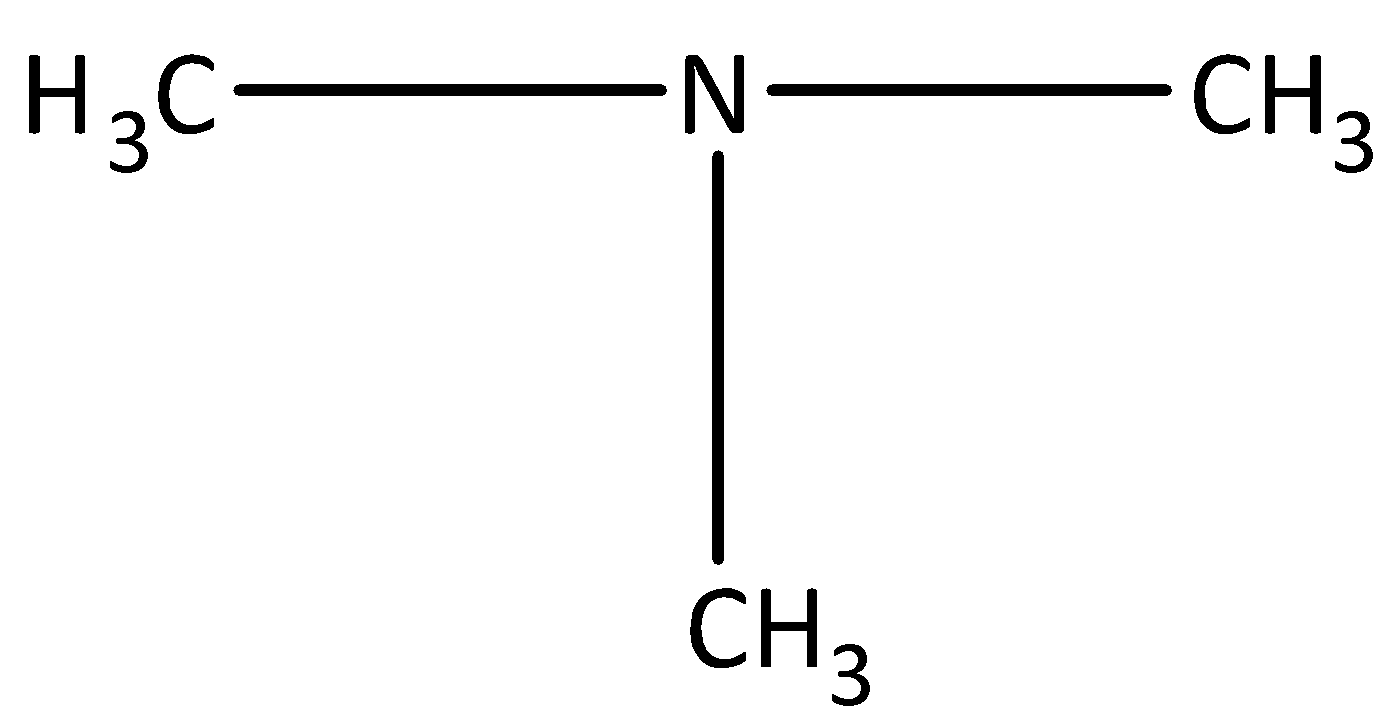
Always remember that, amine is a functional group which is the derivative of ammonia. Amine can be obtained by replacing one, two or all hydrogen atoms of ammonia. If one hydrogen is replaced, the amine is known as primary amine, such as CH3−NH2, if two hydrogen atom is replaced by, then the amine is secondary amine, such as, CH3−NH−CH3 and if all hydrogen atoms are replaced, then the amine is termed as tertiary amine, such as,