Question
Question: Acetaldehyde reacts with \({\text{NaOH}}\) to form: A. 
B. 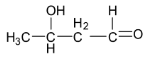
C. 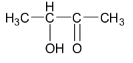
D. 
Solution
The carbon atom next to the carbonyl group in an aldehyde or a ketone is called α - carbon and the hydrogens attached to it are called α - hydrogens. These α - hydrogens are acidic due to the electron withdrawing inductive effect of the carbonyl group and so they can be easily abstracted by strong bases to give enolate ions.
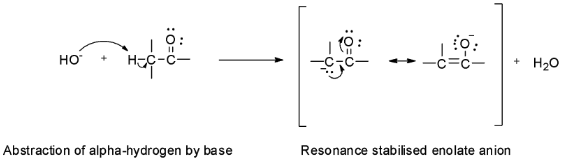
Based on this acidity of α - hydrogens, the aldol condensation reaction involves the reaction of aldehyde or ketone with dilute alkali to form a β - hydroxyaldehyde or a β - hydroxyketone. These β - hydroxyaldehyde or β - hydroxyketone are called aldols.
Complete step by step answer:
Acetaldehyde is an aldehyde containing α - hydrogen atoms and so they can be easily abstracted by a base. So acetaldehyde will undergo aldol condensation reaction with sodium hydroxide to give 3- hyroxybutanal which is an aldol. The reaction is shown below:
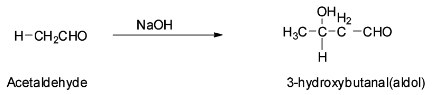
The mechanism of the above reaction is discussed below.
Step 1: Abstraction of acidic alpha hydrogen by the sodium hydroxide base to form an enolate ion.
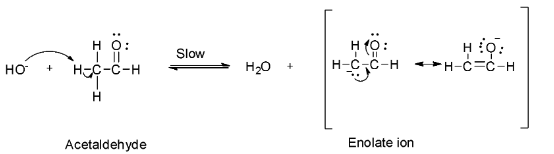
Step 2: Nucleophilic attack of the enolate on the second acetaldehyde molecule to form the anion (I).
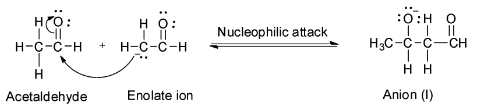
Step 3: Abstraction of proton from water by the anion (I) to form aldol.

Option A is not correct as the given aldehyde structure contains hydroxy group in the alpha position and so it is not a betahydroxyaldehyde or aldol.
Option C is not correct as the given ketone structure contains hydroxy group in the alpha position and so it is not a betahydroxyketone or aldol.
Option D is not correct as the given aldehyde structure contains hydroxy group in the gamma position and so it is not a betahydroxyaldehyde or aldol.
Thus, option B is correct.
Note:
Aldehydes which do not have α - hydrogen atoms will not undergo aldol condensation reaction.
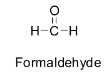
For example, formaldehyde does not contain any α - hydrogen atom. So there are no acidic hydrogens available for abstraction by bases and hence it cannot undergo an aldol reaction to give beta hydroxy aldehyde or aldol. Its structure is shown below.
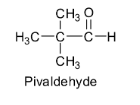
Pivaldehyde also does not contain any α - hydrogen atom. So there are no acidic hydrogens available for abstraction by bases and hence it cannot undergo an aldol reaction to give aldol. Its structure is shown below.