Question
Question: Acetaldehyde reacts with semicarbazide, product will be: A.\[C{{H}_{3}}CH=NNH-CO-N{{H}_{2}}\] B....
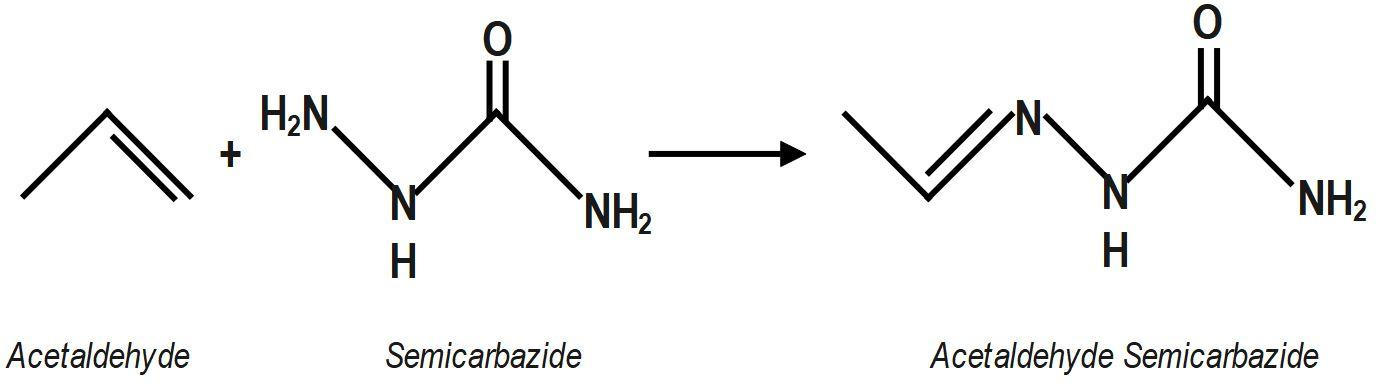
Acetaldehyde reacts with semicarbazide, product will be:
A.CH3CH=NNH−CO−NH2
B.CH3CH=NCONHNH2
C.CH3CH=NHNH2
D.CH3−C(=O)−NH−CONH2
Solution
We know that Semicarbazone are derivatives of imines and formed by the condensation reaction between an aldehyde or ketone and semicarbazide. Chemical formula of semicarbazide is NH2(CONH)NH2 the condensation reaction between acetaldehyde (CH3CHO) and semicarbazide to get the molecular formula of acetaldehyde semicarbazone.
Complete answer:
Acetaldehyde semicarbazone belongs to the group of semicarbazones. Semicarbazones are formed by the condensation reaction between an aldehyde or ketone and semicarbazide. A condensation reaction proceeds with the loss of a water molecule. So, to form acetaldehyde semicarbazone, we need to do the condensation reaction of aldehyde, which is acetaldehyde here, with semicarbazide. Semicarbazide is a derivative of urea.
Some semicarbazones have antiseptic properties. Nitrofurazone (trade name as Furacin) is such an example. Thiosemicarbazone is a semicarbazone which contains sulphur atoms (thio group) in the place of oxygen atoms. It possesses anti-viral, anti-malarial and anti-cancer activities.
Therefore, the correct answer is option A.
Note:
Remember that Semicarbazones are crystalline solids and they are very useful for the identification of aldehydes and ketones by the melting point analysis method. Molecular weight of acetaldehyde semicarbazone is 101 g/mol. Its molecular formula can also be written as: C3H7N3O.
