Question
Question: Acetaldehyde on treatment with aluminium ethoxide gives: (A) ethyl methanoate (B) ethyl acetate ...
Acetaldehyde on treatment with aluminium ethoxide gives:
(A) ethyl methanoate
(B) ethyl acetate
(C) ethyl propionate
(D) ethyl formate
Solution
We are treating acetaldehyde with aluminium ethoxide first let’s understand the purpose of aluminium ethoxide or aluminium triethoxide it is a strong reducing agent which is used in some chemical reaction and it has some industrial applications also. We will see the reaction involved in the above question and try to understand the process behind it.
Complete answer:
The reaction in this question is known as the Tishchenko reaction in which aldehydes undergo a process of dimerization to form esters in the presence of a metal catalyst. This reaction can take place between aldehydes or an aldehyde and a ketone. It is a disproportionate reaction. the reaction for above mentioned question
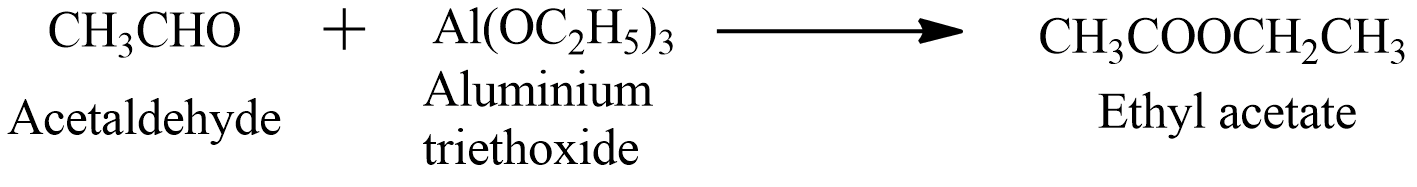
We can see from the above reaction that acetaldehyde reacts with Aluminium ethoxide or Aluminium triethoxide to form ethyl acetate. This reaction is widely used by industries for the production of the ethyl acetate.
Additional Information:
The Tishchenko reaction is generally carried out under very dry atmosphere and inert conditions because of the catalyst used in the reaction which are highly reactive towards hydrolysis cooling is also required as this reaction is exothermic and a side product can be formed if temperature is not according to the requirement of the reaction.
Note:
The Aluminium ethoxide acts as a Lewis acid so it can coordinate with one molecule of aldehyde in addition with the other molecule of aldehyde. Then it forms a hemiacetal as an intermediate product. Aluminium ethoxide acts also as a catalyst increasing the rate of the reaction.
