Question
Question: Acetaldehyde on reaction with Grignard reagent and subsequent hydrolysis yields: (a)- Tertiary alc...
Acetaldehyde on reaction with Grignard reagent and subsequent hydrolysis yields:
(a)- Tertiary alcohol
(b)- Primary alcohol
(c)- Secondary alcohol
(d)- Both primary and secondary alcohol
Solution
The given compound in the reaction is acetaldehyde or ethanal and ethanal is a primary compound. Grignard reagent means there is magnesium, alkyl group, and halogen so, its formula will be RMgX, when this reacts with carbonyl group then there will be the addition of alkyl group at the carbon atom having the oxygen atom.
Complete answer:
In the reaction given in the question, the reactant is acetaldehyde or ethanal because there are two carbon atoms and there is an aldehyde functional group whose formula will be CH3CHO, and this ethanal reacts with Grignard reagent means there is magnesium, alkyl group, and halogen so, its formula will be RMgX. The alkyl group present with MgX in the compound can be methyl, ethyl, propyl, etc.
When this reacts with the carbonyl group then there will be the addition of an alkyl group at the carbon atom having the oxygen atom. Now, when the Acetaldehyde or ethanal reacts with Alkyl magnesium halide, then the alkyl group will attack the carbon atom having the carbonyl group, and the MgX will attack the Oxygen atom.
When this molecule is treated with water then the –OMgX group will be hydrolyzed and it will convert into the –OH group. The series of reaction is given below:
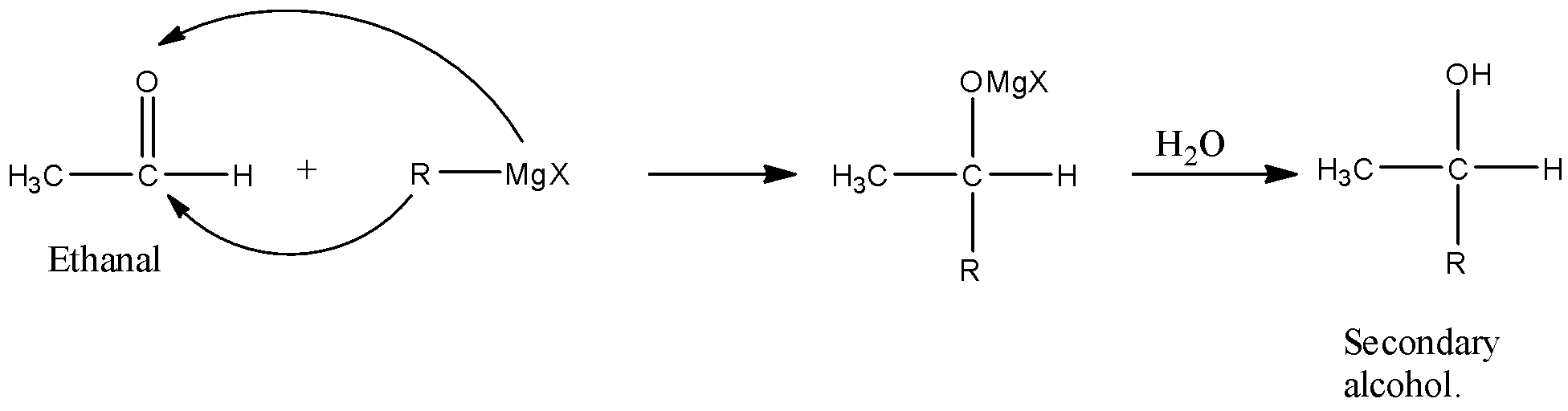
So, the product formed is secondary alcohol because the hydroxyl group is attached with a carbon atom that is further attached with two carbon atoms.
Therefore, the correct answer is an option (c).
Note:
To form primary alcohol by using Grignard reagent then we have to use methanol as reactant because the only methanol can form primary alcohol, and with ketones, always tertiary alcohols are formed.
