Question
Question: Account for the following: A. \({\rm{PC}}{{\rm{l}}_{\rm{5}}}\)is more covalent than \({\rm{PC}}{{\...
Account for the following:
A. PCl5is more covalent than PCl3
B. Iron on reaction with HCl forms FeCl2and not FeCl3
C. The two O−Obond lengths in the ozone molecule are equal.
Solution
We know that the covalent character of a compound depends on the oxidation state of the central metal atom. Iron is a metal and HCl is an acid, so when both react, salt and hydrogen gas is formed and .Ozone is a molecule whose structure is bent and three oxygen atoms present in the molecule.
Complete step-by-step answer:
(i) We have to calculate the oxidation state of P in PCl5andPCl3 to compare the covalent character of the two compounds. More the oxidation state of the central metal atom, greater the polarizing power which results in more covalent character of the bond formed between central metal atom and the other atoms.
Now, we calculate the oxidation state of P inPCl5. The oxidation number of chlorine is -1 and the oxidation number of neutral compounds is zero.
Take x as the oxidation number of Phosphorus (P) in PCl5 .
x−5=0\x=5
Similarly, calculate the oxidation state of P inPCl3.
x−3=0\x=3
So, in PCl5, oxidation state of P is more than PCl3. Hence, PCl5is more covalent than PCl3.
(ii) Now, we write the reaction of Fe and HCl,
Fe+HCl→FeCl2+H2
From the above reaction, we observed that hydrogen gas is evolved. The liberated H2undergoes reaction with oxygen to produce water molecules. The formation of water molecules reduces the oxidation of ferrous chloride to ferric chloride FeCl3. Hence, FeCl3 will not form.
(iii) We know that, in the molecule of ozone, one single bond and one double bond is present.
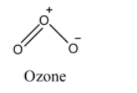
The oxygen atoms of ozone are arranged in such a way that it forms a bent structure. One terminal O atom makes a single bond to the central O atom and the other terminal O atom makes a double bond to the central O atom. The pi electrons of the double bond are delocalized over all the three O atoms in the molecule. Due to this, the bonds (double and single) are not pure and the bonds are resonance hybrids of single and double bonds respectively. So, the bond length of two O-O bonds is equal.
Note: (i) Between PCl5and PCl3, the oxidation state of P in PCl5 is greater than PCl3. This causes PCl5 having more covalent character than PCl3.
(ii) The FeCl3 can be produced by reacting iron with stronger oxidizing agent such as chlorine.
(iii) The resonance effect observed in the ozone molecule results in an equal bond length of the two O−O bonds. So, we can say that resonance affects the bond lengths of molecules.
