Question
Question: According to the MO theory: A.\[{O_2}^ + \] is paramagnetic and bond order is greater than \[{O_2}...
According to the MO theory:
A.O2+ is paramagnetic and bond order is greater than O2
B.O2+ is paramagnetic and bond order is less than O2
C.O2+ is diamagnetic and bond order is less than O2
D.O2+ is diamagnetic and bond order is more than O2
Solution
The bond order is a measure of the number of bonds that exist between two atoms and can be calculated by multiplying the difference of number of electrons present in bonding and antibonding molecular orbitals by half.
Complete answer:
A simple oxygen molecule is diatomic in nature and consists of double bonds. The double bond present in oxygen molecules is an indicator of the fact that its bond order is 2 .
O2+ is chemically different from the simple oxygen molecule O2 as it has lost one electron or it has one electron less as compared to the nitrogen molecule.
The placement of electrons in bonding or antibonding molecular orbitals can be determined by drawing the molecular orbital diagram of the molecule. This diagram is the virtual representation of the linear combination of atomic orbitals that combine to give molecular orbitals.
The molecular orbital diagram of an O2+ ion can be shown as follows:
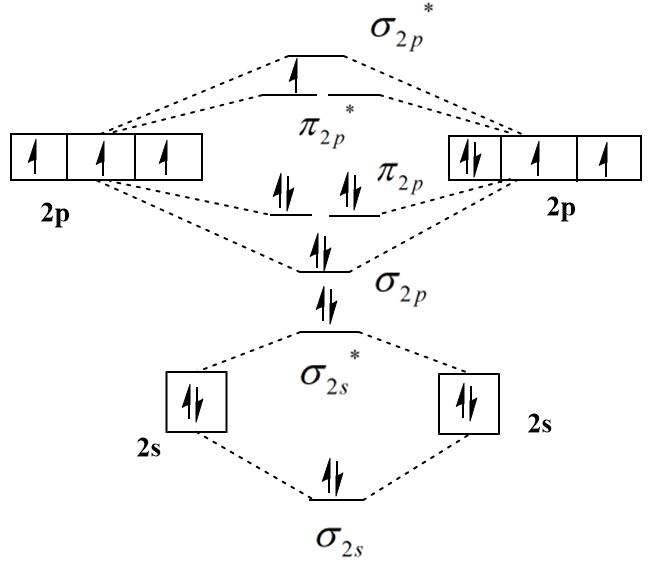
Thus, the outermost electronic configuration of O2+ molecule is σ2s2σ∗2s2σ2p2π2p4σ∗2p1 which indicates that there are a total of eight electrons in bonding molecular orbitals and two electrons in the antibonding molecular orbitals. The following formula can be used to calculate the bond order:
bond order=2bonding electrons−anti - bonding electrons
bond order=28−3=2.5
⇒ Thus, the bond order of O2+ ion is higher than that of O2 molecule and the presence of an unpaired electron in the highest occupied molecular orbital (HOMO) makes it paramagnetic.
Therefore option (A) is correct.
Note:
The star marked molecular orbitals are the anti-bonding molecular orbitals that are formed due to the destructive overlap that takes place between atomic orbitals. The electrons present in anti-bonding molecular orbital do not contribute in the bond formation and are therefore subtracted.
