Question
Question: According to octet rule, \(S{{O}_{3}}\) contains_______ dative bonds A.1 B. 2 C.3 D.4...
According to octet rule, SO3 contains_______ dative bonds
A.1
B. 2
C.3
D.4
Solution
In terms of electron-counting method, sulphur atom has an oxidation state of +6 and a formal charge of 0. According to the Lewis structure SO3 consists of S=O double bond and two S–O dative bonds without utilizing d-orbitals.
Complete answer:
Let us know about octet rule, to answer this question. According to the Octet rule, all the elements tend to form their bonds in such a way that they have 8 electrons in their outermost shell. The outermost shell can contain a maximum of 8 electrons and if an element is able to achieve it, then the element attains maximum stability and it is called noble gas configuration. So, during formation of bonds, sharing of electrons occurs so that both the participating elements fill up their outermost or valence shell.
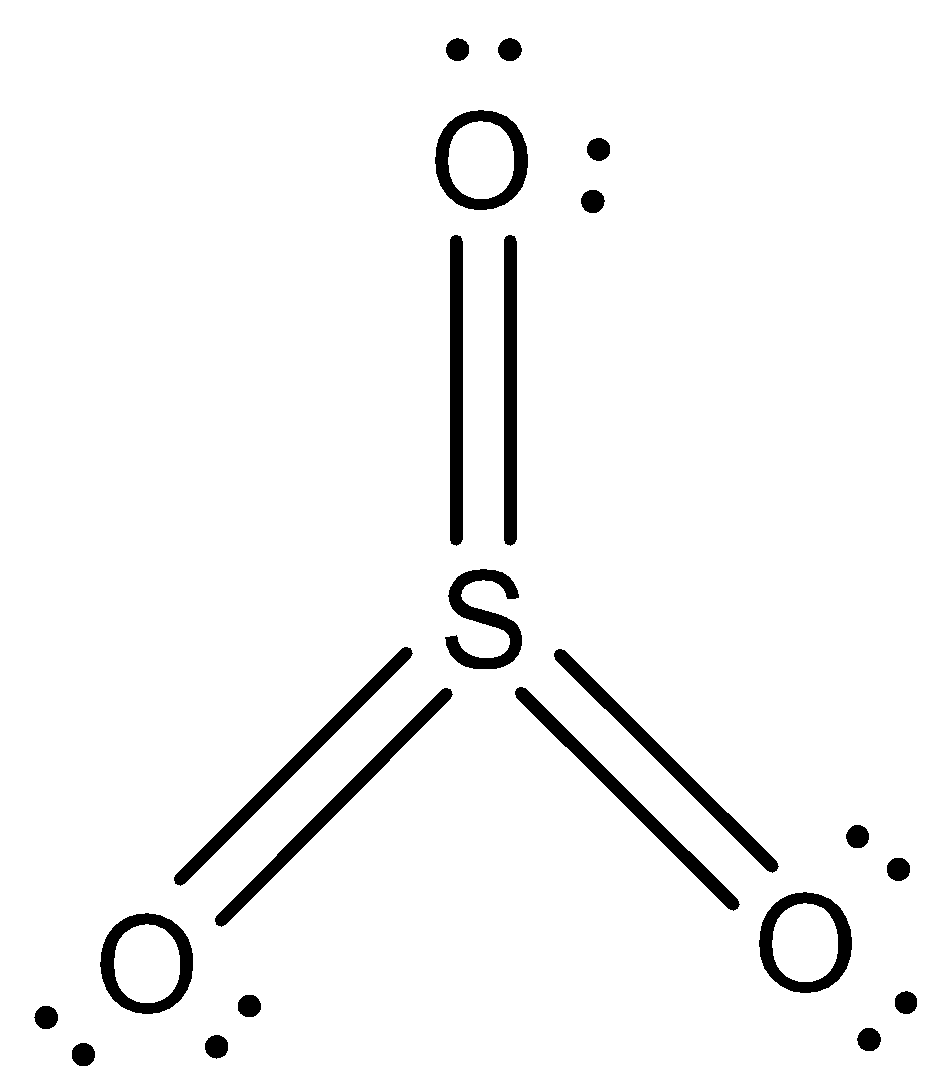
This is the structure of SO3 where each element achieves octet stability
Valence electrons in Sulphur(S)=6
Total no of valence electrons=6+3×6=24
According to the octet rule we can say that the Lewis structure consist of an double bond (S=O) and two dative bonds to complete its octet as:
Two dative bonds , 2 bond×2electrons = 4
One double bond , 2×2electrons = 4
together containing 8 electrons.
Structure of the gas state SO3 is a trigonal planar molecule of D3H symmetry, as predicted by the famous VSEPR theory. The SO3 belongs to D3H point group.
The electrical dipole moment of the gaseous sulphur trioxide is zero. This is a consequence of 1200 angle between the S-O bonds.
Sulphur trioxide can be found as colorless to white crystalline solid which will fume in air as colorless liquid and gas. It has a molecular weight of about 8.066gmol−1. It has a density of 1.92gcc−1in liquid form.
Melting point of Sulphur trioxide is 16.90C(62.40F; 290.0 K) and boiling point is 450C (1130F; 318 K)
Note:
In SO3 there are a total of 6 covalent bonds in which there are 3−σbonds and 3−π bonds. There are no co-ordinate covalent bonds present because no bond in SO3 is formed by donating an electron pair. So, the total number of coordinate bonds present in SO3 molecule is zero.
