Question
Question: According to Lewis dot structure the number of bond around central atom is greater than four for whi...
According to Lewis dot structure the number of bond around central atom is greater than four for which of the following anion:
(A) CO3−2
(B) NO3−
(C) CO4−3
(D) none of the above
Solution
Lewis structure is drawn by taking valence electrons in account. The valence electrons are drawn around the atom, and sharing of electrons between the atoms is shown. Single bonds have two electrons shared and double bonds have 4 electrons shared between.
Complete step by step answer: Lewis structure, also known as Lewis Dot diagram, lewis dot structures, electron dot structures or lewis electron dot structures, are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds. The Lewis structure was named after Gilbert N. Lewis, who introduced this concept. Now we will draw the lewis structure of each of the options given, to know which option has more than four bonds around it. The structure of CO3−2 is as follows,
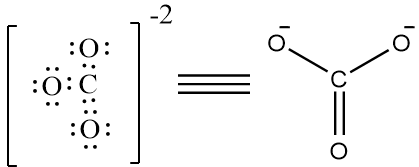
There are 4 bonds present. Therefore, it is not the correct option. The structure of NO3− is as follows,
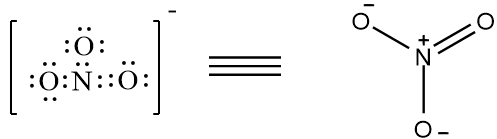
The compound has 4 bonds present. Therefore it is not the correct option. The compound CO4−3 does not exist, as carbon can’t have more than 4 bonds. So, none of the above given options have greater than four bonds around the central metal atom.
Hence, the correct answer is the D option.
Note: Lewis structure shows each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another. Excess electrons that form lone pairs are represented as pairs of dots, and are placed next to the atoms.
