Question
Question: According to J. Chadwick, ______________ is not found in hydrogen atoms. A. neutron B. electron...
According to J. Chadwick, ______________ is not found in hydrogen atoms.
A. neutron
B. electron
C. proton
D. none of these
Solution
Hydrogen atoms have atomic number 1 , so that means it will have electrons. In order for the atom to be electrically neutral, it will also need to have protons. Since Hydrogen also has the smallest nucleus, there is no space for any additional species in the nucleus.
Complete step by step answer:
The atom of hydrogen looks like this: 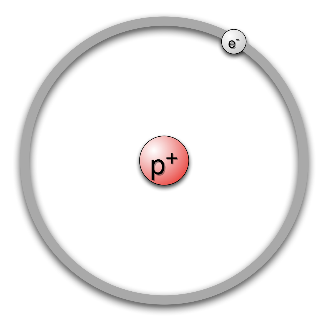
Hydrogen has the atomic number 1 , which means that it will need to have one electron in its orbit. So, we can see in the diagram, that there is one electron orbiting around the nucleus of the atom.
Now, we know that hydrogen has to be electrically neutral. Hence, it needs to have a charge opposite to the electron so the net charge on the atom is zero.
We have considered the electron and protons of the atom. Now, since the nucleus of the atom is much smaller, there is no space for any further inclusions. Hence, a neutron, which is a neutral species, will not be part of the atom model. Hence we can see in the diagram, that the electron revolves around only a proton center.
Hydrogen has three isotopes, which means 3 species, with the same atomic number, but different neutron-proton ratio. The Nucleus discussed above, belongs to protium , which is the most abundant species of hydrogen in the atmosphere. Deuterium has 1 proton and 1 neutron in the nucleus. Tritium has 2 neutrons and 1 proton in the nucleus.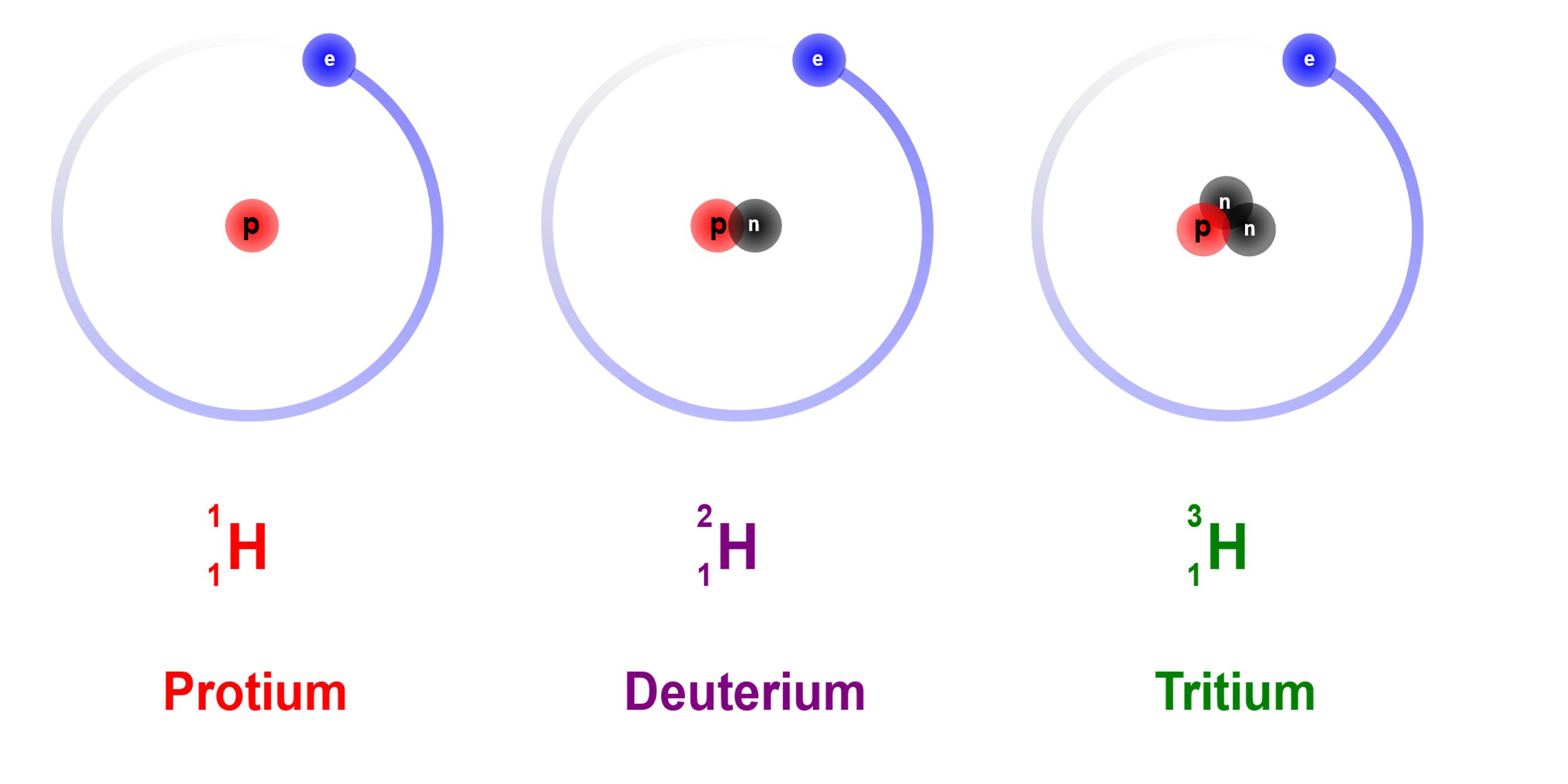
They all have the same number of electrons since they have the same atomic number. Hence neutrons are not found in hydrogen atoms.
So the correct answer is option” A”.
Note:
It is important to note that the neutron was first theorized by Ernest Rutherford in the year 1920 (when he did his experiments on protons) but he had no proof for his theory. J. Chadwick later discovered neutrons by using polonium as the source of neutrons. This proved to be a milestone discovery in the field of nuclear science.
