Question
Chemistry Question on Equilibrium
According to Bronsted-Lowry concept of acids and bases a conjugate acid-base pair can exist as
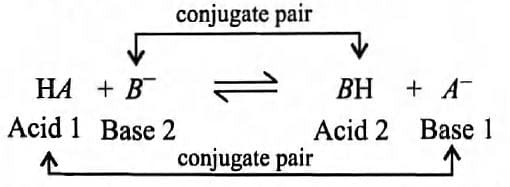
Mark the option in which conjugate pair is not correctly matched.
Species Conjugate acid Conjugate base
A
HCO3−CO32−H2CO3
B
HPO42−H2PO4−PO43−
C
NH3NH2−NH4+
D
HS−S2−H2S
Answer
HPO42−H2PO4−PO43−
Explanation
Solution
Brønsted-Lowry acid-base conjugate pairs differ by just one proton. Brønsted-Lowry acids are proton donors, while Brønsted-Lowry bases are proton acceptors. The conjugate acid of hydrogen phosphate HPO42− is dihydrogen phosphate H2PO4−, and its conjugate base is phosphate PO43−.
