Question
Question: Accomplish the following conversions: (i) Nitrobenzene to benzoic acid...
Accomplish the following conversions:
(i) Nitrobenzene to benzoic acid
Solution
The answer to this question is based on the basic concepts of organic chemistry which includes the reagents used for particular conversions and also the type of reaction that is whether oxidation, reduction or substitution reaction.
Complete step by step answer:
In our classes in organic chemistry, we have studied the named reactions and also several common reactions that include addition reaction, substitution reactions, elimination reactions and so on.
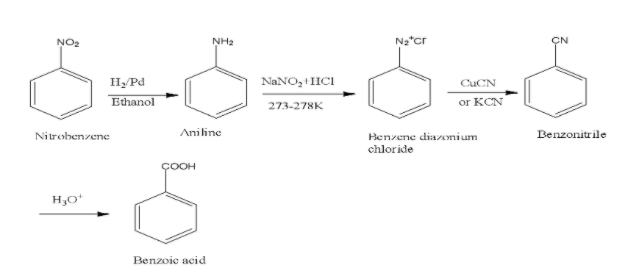
To convert nitrobenzene to benzoic acid, firstly we must know that nitro group is electron withdrawing group and thus it is reduced in first step to aniline with amine group in it in the presence of reducing agent that is H2/Pd and then converting it into diazonium salt which when treated with copper cyanide and subsequent hydrolysis yields benzoic acid. The reaction is shown below,
Note: Note that several common oxidising and reducing agents are to be remembered and based on this you will be able to solve any type of such question that involve conversions from one compound to another.
