Question
Question: $A \xrightarrow{NH_2OH} B \xrightarrow{PCl_5} C \xrightarrow{H_2O} HOOC-(CH_2)_4NH_2$ $A + PhCHBrCO...
ANH2OHBPCl5CH2OHOOC−(CH2)4NH2
A+PhCHBrCOOEtbaseD, Compound D is?
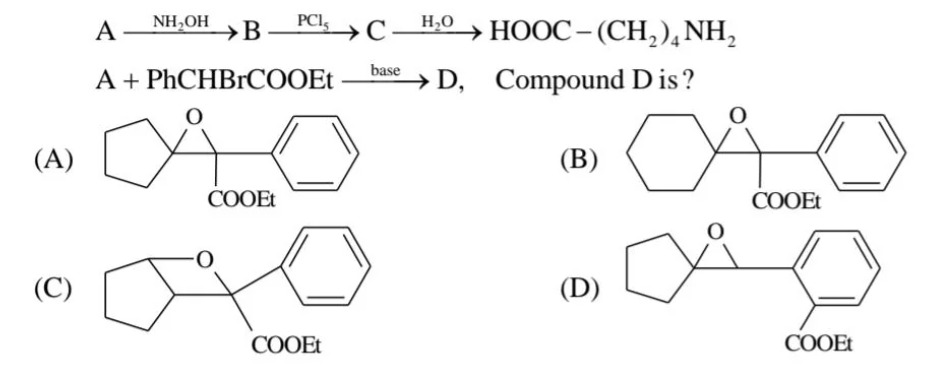
A cyclopentane ring is connected to a carbon atom. This carbon atom is connected to an epoxide ring (oxygen atom connected to the carbon atom of the cyclopentane ring) and a phenyl group. The same carbon atom is also connected to a COOEt group (ethyl ester).
A cyclohexane ring is connected to a carbon atom. This carbon atom is connected to an epoxide ring (oxygen atom connected to the carbon atom of the cyclohexane ring) and a phenyl group. The same carbon atom is also connected to a COOEt group (ethyl ester).
A cyclopentane ring is connected to a carbon atom. This carbon atom is connected to an oxygen atom and a phenyl group. The same carbon atom is also connected to a COOEt group (ethyl ester).
A cyclopentane ring is connected to a carbon atom. This carbon atom is connected to an epoxide ring (oxygen atom connected to the carbon atom of the cyclopentane ring) and a phenyl group with a COOEt group (ethyl ester) at the ortho position.
Option (D)
Solution
-
The first reaction sequence converts A into HOOC–(CH₂)₄–NH₂ via oxime formation, rearrangement (using PCl₅) and hydrolysis. This shows that A is a cyclopentanone‐derived ester.
-
In the second reaction, A reacts with PhCHBrCOOEt in the presence of base. The resulting substitution and rearrangement is analogous to a benzilic acid rearrangement where the bromine‐substituted benzyl unit becomes attached to the aromatic ring in an “ortho” fashion.
-
Comparing the given options, only the structure labeled (D) shows a cyclopentane with an epoxide functionality at the alpha‐carbon and a phenyl group bearing a COOEt substituent at the ortho position—exactly as expected for compound D.
