Question
Question: (a) Write two differences between physical adsorption and chemical adsorption. (b) Define homogeno...
(a) Write two differences between physical adsorption and chemical adsorption.
(b) Define homogenous and heterogenous catalysis. Give an example of each.
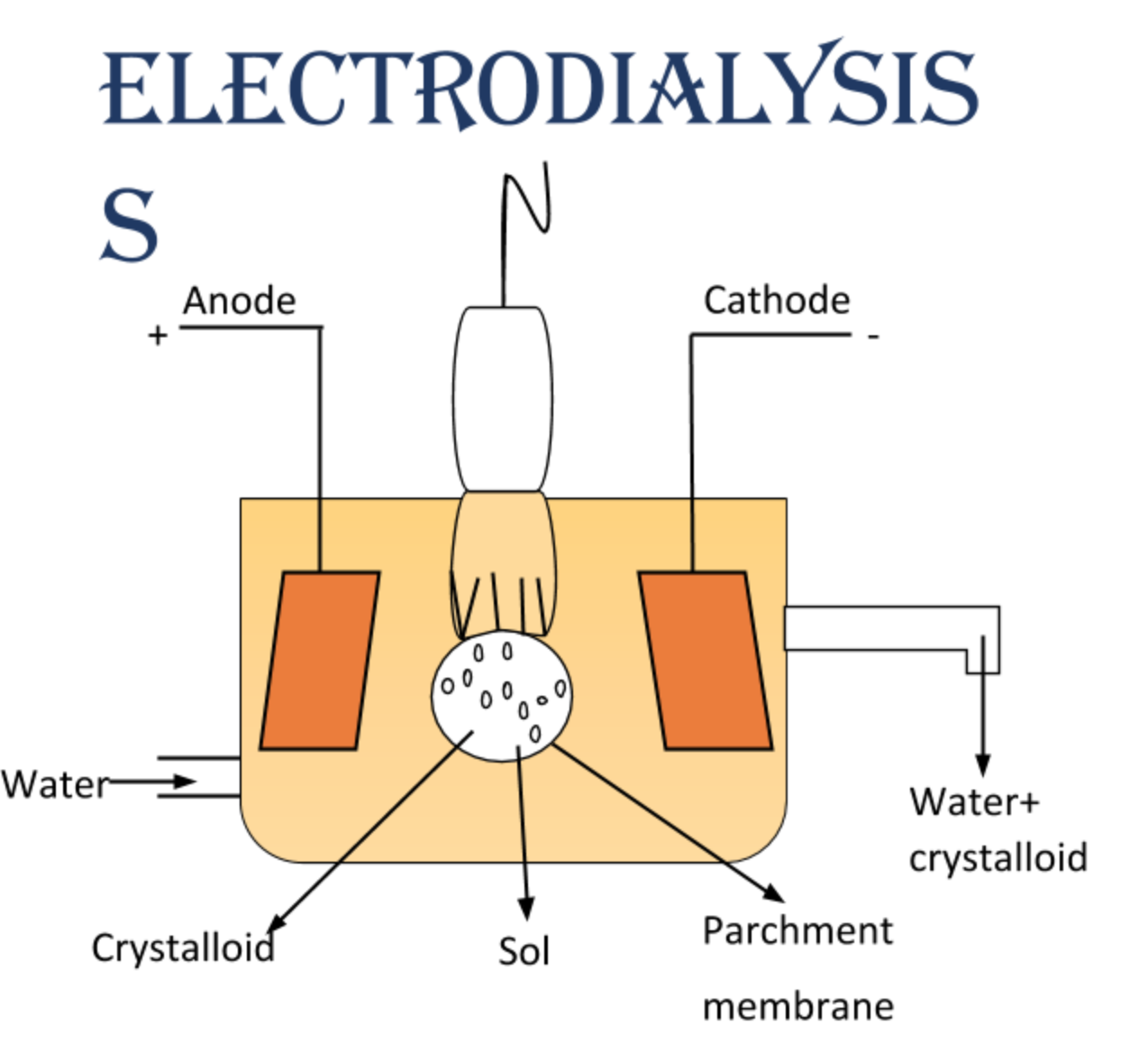
(c) Draw labelled diagram of electro-dialysis.
Solution
. Physical and chemical adsorption arises because of the Vander waals' forces and chemical bonds respectively and are reversible or irreversible in nature and in case of homogenous and heterogenous catalysis the difference lies only in the phases of the reactants and the products. Electro means electricity and dialysis means purification , so, electrodialysis is the process of purification by applying the electric field and consists of a battery, a membrane and colloidal solution. Now answer the given statements accordingly.
Complete step by step answer:
Considering the given statement one by one as;
(a) By adsorption, we mean the phenomenon of the retention of the molecules of the substance on the surface which can be either solid or liquid. It is of two types:- physical and chemical adsorption.
We will differentiate between the physical and chemical adsorption in tabular form because it’s easy to compare them in tabular form.
| Sr.no | Physical adsorption | Chemical adsorption |
|---|---|---|
| 1. | It arises when the particles of adsorbate ( the substance which is adsorbed) are held by weak Vander waals' forces. | It arises when the particles of adsorbate ( the substance which is adsorbed) are held by the simple chemical bonds. |
| 2. | It can be reversed either by increasing temperature or by decreasing pressure. | It is an irreversible process. |
(b) Homogenous catalysis is that catalysis in which the reactants and products are present in the same phase i.e. either in solid, liquid or gas.
Example:- In the hydrolysis of ester. The reaction occurs as:
CH3COOC2H5(l)+H2O(l)heatCH3COOH(l)+C2H5OH(l)
Heterogeneous catalysis is that catalysis in which the reactants and products are present in the different phases i.e. either in solid, liquid or gas.
Example:- 2SO2(g)+O2(g)V2O52SO3(g)
(c) By electro dialysis we mean the separation of the particles of colloids from those of crystalloids by applying the electric field through a suitable membrane. The diagram of electrodialysis is as:
Note: In physical adsorption, no activation energy is needed but in case of chemical adsorption, high activation energy is required. Activation energy is the minimum energy which is required by a chemical reaction to occur.
