Question
Question: (a)Write the products formed when \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\]reacts with the f...
(a)Write the products formed when CH3CHOreacts with the following reagents:
(i) HCN
(ii) H2N - OH
(iii) CH3CHOin the presence of diluteNaOH
(b) Give the simple chemical tests to distinguish between the following pair of compounds
(i) Propanal and propanone
Solution
Hint- we must remember that the CH3CHO known as acetaldehyde and has aldehyde as the functional group. The carbon-oxygen double bond of - CHOis polarised due to higher electronegativity of oxygen relative to carbon. Therefore we can understand the carbonyl oxygen is a nucleophilic (Lewis base) centre and the carbonyl carbon is an electrophilic (Lewis acid) and which can undergo nucleophilic addition reactions.
We must understand that the propanal is an aldehyde and propanone is a ketone, so we need to find a test to distinguish between aldehyde and ketone. Therefore we can use Iodoform test to inspect the presence of carbonyl compounds with the structure R - CO - CH3or alcohols with the structureR - CH(OH) - CH3.
Complete step by step solution:-
(a) (i) when we react aldehydes and ketones with hydrogen cyanide (HCN) which yield cyanohydrins. So in the given question, acetaldehyde (CH3CHO) reacts with hydrogen cyanide HCN to give 2-hydroxypropanenitrile as product. We can represent the reactions as
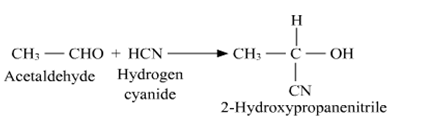
(ii) When we reacts acetaldehyde (CH3CHO) with hydroxylamine (H2N - OH) to give acetaldoxime (oxime) as a product.
CH3CHO + NH2OH→CH3 - CH = NOH + H2O
(iii) Also the reaction of acetaldehyde (CH3CHO) with acetaldehyde (CH3CHO) in the presence of diluteNaOH, we know this is a type of aldol reaction by which we obtained 3-hydroxybutanal as product. Further, we heat the reaction mixture, we get aldol condensation product (but-2-enal).
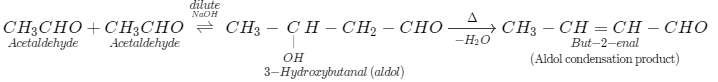
(b) We can use the Iodoform test to distinguish propanal and propanone. We must understand that when we treat this compound with iodine in the presence of base likeNaOH, we get a pale yellow precipitate of triiodomethane (iodoform) for carbonyl compounds with the structure R - CO - CH3or we get alcohols with the structureR - CH(OH) - CH3. Now we can distinguish, propanone has R - CO - CH3structure gives positive result with iodoform while propanal do not contain any such structure gives negative result.
Note- (a) we must know that all the reactions of acetaldehyde with corresponding reactants is a result of polarised C-O bonds in the aldehyde.
(b) We must look for carbonyl compounds with the structure R - CO - CH3or alcohols with the structure R - CH(OH) - CH3in order to carry out iodoform test.
