Question
Question: (a) Write the mechanism of the following reaction: \(2\,{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}...
(a) Write the mechanism of the following reaction:
2CH3CH2OH→H+ CH3CH2−O−CH2CH3
(b) Write the equation involved in the acetylation of salicylic acid.
Solution
The ester can be prepared from the alcohol by protonation followed by SN2 attack of another molecule of alcohol and then deprotonation. Acetylation of salicylic acid takes place in presence of acetic anhydride and sulphuric acid.
Complete Step by step answer: (a)
Two molecules of propanol forms ethoxyethane in presence of acid. We have to determine the mechanism of the reaction.
The reaction is as follows:
2CH3CH2OH→H+ CH3CH2−O−CH2CH3
The complete reaction is take palce in three steps:
Step-1: Protonation of ethanol.
The oxygen atom of ethanol has lone pair so, it attacks on proton and get protonated so, a positive charge is generate on oxygen of ethanol.
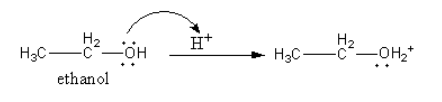
Step-2: SN2 attack of another molecule of ethanol.
The water molecule removes from the protonated ethanol simultaneously the oxygen atom of another ethanol molecule attacks from other side.
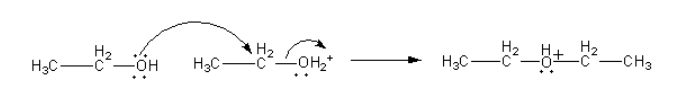
Step-3: Deprotonation from ether.
The hydroxyl ion of water molecules attacks on positively charged oxygen atoms to remove protons and give ether.
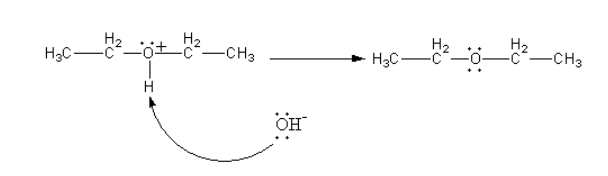
Therefore, the ester from alcohol is formed by protonation, SN2attack of second molecule of ethanol followed by deprotonation.
(b)
The equation involved in the acetylation of salicylic acid.
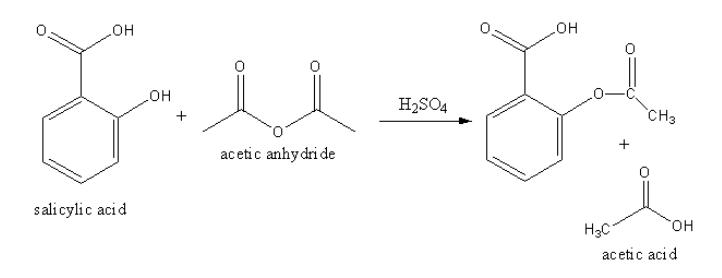
The acetylation of salicylic acid takes place in presence of strong acid like sulphuric acid.
The salicylic acid reacts with acetic anhydride in presence of sulphuric acid to form acetylated salicylic acid and acetic acid.
Note: The full name of SN2reaction is a bimolecular nucleophilic substitution reaction. The SN2reaction removal of a nucleophile and the attack of another nucleophile take place simultaneously. The compound with less steric hindrance will give the SN2 reaction faster.
