Question
Question: a) Write the mechanism of the following reaction  Write the mechanism of the following reaction

b) Write the equation involved in Riemer-Tieman reaction.
Solution
Nucleophiles generally takes part in the nucleophilic substitution reactions and during this reaction nucleophile becomes attracted towards a partial or full positive charge rather than this neutral nucleophilic reactions with solvents like water is known by the name solvolysis.
Complete answer:
Nucleophilic substitution reaction is generally of two types known by the name SN1and SN2i.e. unimolecular nucleophilic substitution and bimolecular nucleophilic substitution.
a) The mechanism of first reaction is based on the mechanism of SN2nucleophilic substitution this mechanism is known by the name bimolecular nucleophilic substitution and it is a single step reaction and one transition state is shown in this. Firstly nucleophilic attack on the compound then its transition state the attack of nucleophile and leaving of other group will be seen and then leaving group will leave the compound. In the reaction given in the question attack of bromide ion and removal of oxide ion can be seen as follows:
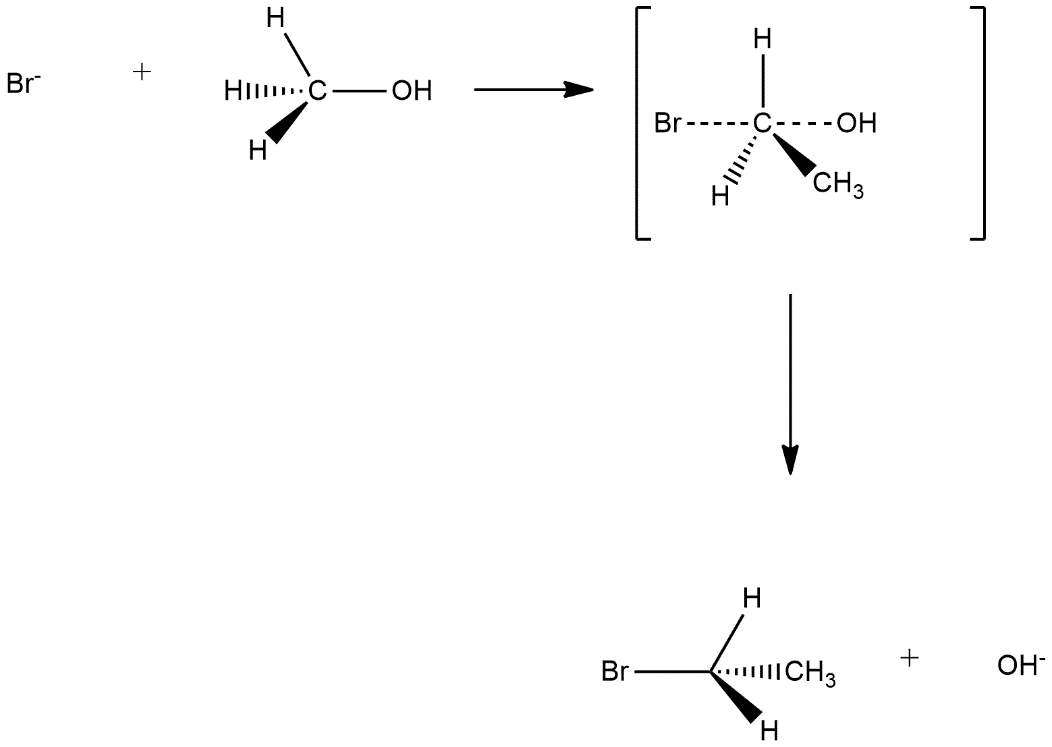
We can observe that in this reaction inversion takes place.
b) Write the equation involved in Riemer-Tieman reaction:
To write the Riemer-Tieman reaction we can take the example of benzoyl which reacts with chloroform and sodium hydroxide and give the major product salicylaldehyde and the reaction can be shown as follows:
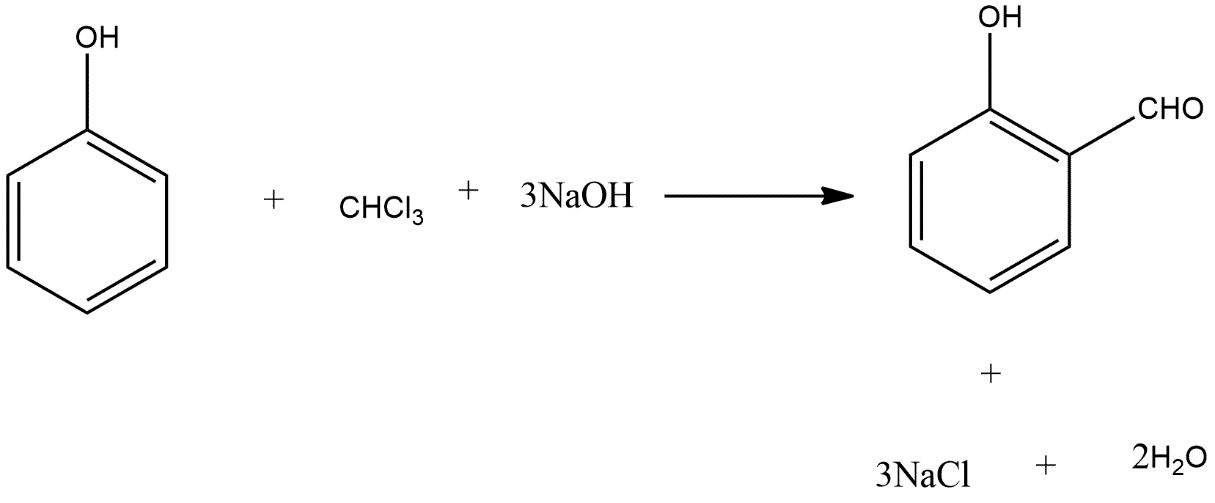
Hence this is the main example of Riemer-Tieman reaction.
Note:
Retention refers to that phase of the molecule in which the molecular composition is preserved throughout the reaction whereas inversion refers to that mechanism in which the structure of the molecules is changed during the reaction.
