Question
Question: (a)- Write the IUPAC name of: \([Co{{(N{{H}_{3}})}_{4}}({{H}_{2}}O)Cl]C{{l}_{2}}\) (b)- Explain l...
(a)- Write the IUPAC name of: [Co(NH3)4(H2O)Cl]Cl2
(b)- Explain linkage isomerism with example.
Solution
[Co(NH3)4(H2O)Cl]Cl2 is a coordination compound and in this compound, the coordination complex is the positive part and the chloride ions are the negative part. The oxidation state of cobalt in this compound will be +3. If the coordination complex has an ambidentate ligand then the complex will show linkage isomerism.
Complete Solution :
(a)- Some rules that must be followed while naming the coordination complex. Step by step method is given below:
- In the IUPAC name, the positive ion must be named first and then the negative ion must be named. The compounds written in the complex part must be written in one word without any gap. There are some numerical prefixes used like for 2 it is di, for 3 it is tri, for 4 it is tetra, and these must be used without the hyphen.
- The names of ligands must be written in the alphabetic order even if they are negative, positive, or neutral.
The name of the central metal ion must be written after the name of ligands.
Compounds having complex as a cation, first, write the ligand name, then the central metal ion with its oxidation state and then the name of the anion.
- Complex having complex as an anion, first, write the name of cation and then the name of ligand and to the central metal atom add –ate along with its oxidation state.
Now by following all the rules, the name of the complex [Co(NH3)4(H2O)Cl]Cl2 will be:
In [Co(NH3)4(H2O)Cl]Cl2, the oxidation state of cobalt will be:
x+4(0)+0+(−1)+2(−1)=0
x=+3
The oxidation state is +3 or (III). The name will be Tetraammineaquachlorocobalt (III) chloride.
(b)- In coordination complexes, the metal ion is attached to several ligands, so there are some ligands in which more than one atom in a unidentate ligand can act as a donor. These ligands are known as ambidentate ligands. So when an ambidentate ligand is attached with the central metal ion then it leads to linkage isomerism. For example, nitro molecule (NO2−) have two donor atoms nitrogen and oxygen, its complex will be:
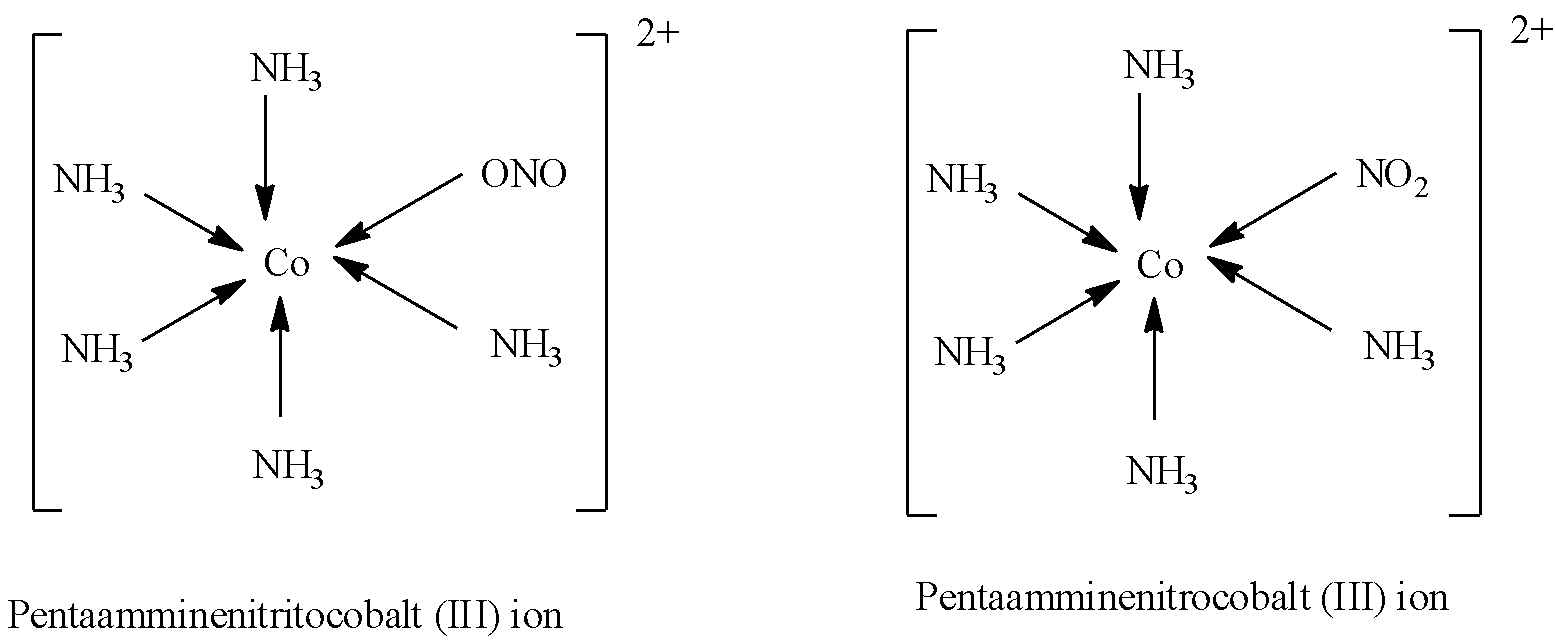
Note: It must be noted that when amines are named only one ‘m’ is used, but when the ligand ammonia (NH3) is named, the 'ammine' term is used. You can also use bis and tris instead of di and tri in the naming.
