Question
Question: (a) Why are axial bonds longer than equatorial bonds in \( PC{l_5}\,? \) (b) What is the hybridiz...
(a) Why are axial bonds longer than equatorial bonds in PCl5?
(b) What is the hybridization of S− atom in SF6 molecule ?
Solution
Hint : Orbital hybridisation is the concept of mixing atomic orbitals into new degenerate hybrid orbitals suitable for the pairing of electrons to form chemical bonds in valence bond theory. For the first question find out the hybridization of the molecule and draw the structure in order to find out the reason for longer axial bonds.
Complete Step By Step Answer:
(a) The electronic configuration of central atom of PCl5 i.e. P is [Ne]3s23p3 , hence P has five electrons in its valence shell. So P can form five bonds at most and hence forms five sigma bonds with the five Cl atoms. In order to form the five sigma bonds one electron from the s orbital is promoted to the d orbital and mixing of orbitals takes place. Hence P has sp3d hybridization as one s , three p and one d orbital are involved in formation of bond. As the molecule has sp3d hybridization it assumes a trigonal bipyramidal geometry.
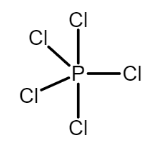
Now, the sp3d hybridization of P can be thought to be composed of (sp2+pd) hybridization i.e. the trigonal bipyramidal geometry is composed of trigonal planar and linear geometry.
So the axial bonds assume a linear geometry and have a pd hybridization while the equatorial bonds aasume a trigonal planar geometry having sp2 hybridization.
We know, more is the s character, more will be the electronegativity and the bonds will be pulled closer to the central atom hence having shorter bond length.
As the equatorial bonds have more s character compared to axial bonds they are pulled closer to P atom and hence are shorter compared to the axial bonds.
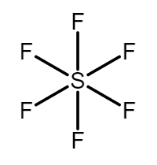
(b) The electronic configuration of central atom of SF6 i.e. S is [Ne]3s23p4 , hence S has six electrons in its valence shell. So S can form six bonds at most and hence forms six sigma bonds with the six F atoms. In order to form the six sigma bonds two electrons one each form the s orbital and the p orbital is promoted to the d orbital and mixing of orbitals takes place. Hence S has sp3d2 hybridization as one s , three p and two d orbitals are involved in formation of bonds. As the molecule has sp3d2 hybridization it assumes an octahedral geometry.
Note :
For both the questions the most important step is to find out the hybridization of the central atom of the molecule. For finding out the hybridization of the central molecule you must know the electronic configuration of the atom properly and for that you must know the periodic table.
