Question
Question: a. What type of isomerism is shown by the complex \[{\left[ {{\rm{Co}}{{\left( {{\rm{N}}{{\rm{H}}_{\...
a. What type of isomerism is shown by the complex [Co(NH3)5(SCN)]2+?
b. Why is [NiCl4]2+ paramagnetic while [Ni(CN)4]2− is diamagnetic? (Atomic number of Ni=28)
c. Why are low spin tetrahedral complexes rarely observed?
Solution
The given complex, [Co(NH3)5(SCN)]2+ has a ambidentate ligand. Paramagnetic complexes have unpaired electrons, whereas diamagnetic ones have paired electrons. Low spin complexes are formed by strong field ligands.
Complete answer:
a. We have seen different types of isomerism in coordination compounds, namely, geometrical and optical; linkage, coordination, ionization and solvate. Let’s have a look at the given complex, [Co(NH3)5(SCN)]2+.
In this complex, there are two different types of ligands, five ammonia molecules and one SCN. Now, we know that SCN is an ambidentate ligand that means it can bind either using its sulphur atom or its nitrogen atom. This is what gives us two different linkage isomers that we can define as those isomers that have same chemical formula but in which an ambidentate ligand is bound using different atoms. For the given complex, [Co(NH3)5(SCN)]2+we will have two linkage isomers as, [Co(NH3)5(SCN)]2+ and [Co(NH3)5(NCS)]2+.
Hence, [Co(NH3)5(SCN)]2+ shows linkage isomerism.
b. In both the given complexes of nickel, we have Ni2+. Let’s write its electronic configuration as follows:
{\rm{Ni}}\left( {Z = 28} \right):\left[ {{\rm{Ar}}} \right]3{d^8}4{s^2}4{p^0}\\\ {\rm{N}}{{\rm{i}}^{2 + }}:\left[ {{\rm{Ar}}} \right]3{d^8}4{s^0}4{p^0} \end{array}$$ Now, in case of $${\left[ {{\rm{NiC}}{{\rm{l}}_4}} \right]^{2 + }}$$, geometry is tetrahedral and thus the hybridization is $$s{p^3}$$that means $4s$ and $4p$ orbitals are used in the hybridization whereas all the eight electrons are in $3d$ orbital as 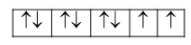 As we can see there are unpaired electrons present and thus it is paramagnetic in nature. On the other hand, in case of $${\left[ {{\rm{Ni}}{{\left( {{\rm{CN}}} \right)}_4}} \right]^{2 - }}$$, geometry is square planar and thus the hybridization is $$ds{p^2}$$ that means $3d,4s$ and $4p$ orbitals are used in the hybridization, whereas all the eight electrons are in $3d$ orbital as 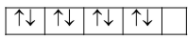 As we can see there are paired electrons present and thus it is diamagnetic in nature. c. We can have two types of complexes namely low spin complexes and high spin complexes. Let’s try to understand how these are formed in tetrahedral compounds on the basis of crystal field splitting. We know that tetrahedral field splitting gives us $e{\rm{ and }}{t_2}$ energy levels and we can represent it as ${\Delta _t}$. Now, it is the comparability of this splitting energy and the pairing energy. If splitting energy is more than the pairing energy due to strong field ligands, then electrons will get paired and we will get low spin complexes. However, if splitting energy is less than the pairing energy due to weak field ligands then electrons will occupy ${t_2}$ level and we will get high spin complexes. Now we know that tetrahedral field splitting, ${\Delta _t}$ is less and electrons can easily remain unpaired in ${t_2}$ level. Hence, we will rarely observe low spin tetrahedral complexes. **Note:** We can relate the octahedral field splitting and the tetrahedral field splitting as ${\Delta _t} = \dfrac{4}{9}{\Delta _o}$ when we have same metal attached to the same ligands with equal metal-ligand distances and see that tetrahedral splitting energy is lesser than the octahedral one.