Question
Question: (a) What is the selectivity of catalysts? Write an example. (b) What happens when hydrated ferric...
(a) What is the selectivity of catalysts? Write an example.
(b) What happens when hydrated ferric oxide sol is mixed with arsenious sulphide sol?
(c) Explain the Tyndall effect with a labelled diagram.
Solution
(a) Selectivity as the name suggests is giving a particular product of the reaction. The reaction may result in different products, but selectivity is a measure key to get the single and desired product. The carbon monoxide can react with hydrogen gas to various products based on the catalyst.
(b) The phenomenon of the precipitation of a colloidal solution by the addition of an excess of an electrolyte is coagulation. There are various methods of coagulation. When two oppositely charged sols are mixed in equimolar proportions, they mutually neutralize their charge and both get coagulated.
(c) When a beam of light passes through the true solution, the path of light is not visible. However, if the light is passed through the colloidal solution, the path of the light becomes visible by scattering the light by colloidal particles. This is a Tyndall effect.
Complete step by step answer:
(a) Catalysts are highly specific compounds. They can direct the reaction to yield a particular product. The reaction with the same reactants but different catalysts may yield different products. This is termed as the selectivity of catalysts.
Let us see some examples, where different products are obtained when different catalysts are used.
CO + 3H2 !! !! !! !! Ni !! !! CH4 + H2O
When carbon monoxide and hydrogen is reacted, with nickel as the catalyst, it produces methane and water.
CO + 2H2 !! !! Cu / ZnO - Cr2O3 !! !! !! !! !! !! CH3OH
When carbon monoxide and hydrogen are reacted, in the presence of copper or zinc oxide or chromium (III) oxide, the product that is formed is methanol.
CO + H2 !! !! !! !! Cu !! !! HCO
When carbon monoxide and hydrogen are reacted, in the presence of only copper, the product is hydrogen carbonate ion.
As we can see that the reactants in all the three cases are the same. But the catalysts are different, and so are the products.
When a hydrated ferric oxide solution is mixed with arsenious sulphide solution, both the solutions are coagulated. Both the solutions, ferric oxide solution, and arsenious sulphide solution have opposite charges. The ferric oxide Fe(OH)3 is a positively charged solution and arsenious sulphide As2S3 when they are fixed, their changes are neutralized. This results in mutual coagulation.
Tyndall effect is defined as the phenomenon of scattering of light by colloidal particles and making the path of light visible through the dispersion. Some examples of the Tyndall effect are: Sunlight entering a dark room and lots of dust particles suspended in the room, when the weather is foggy and smoggy, the beam of headlights is visible and scattering of light by water droplets in the air.
Here is the diagram of the Tyndall effect.
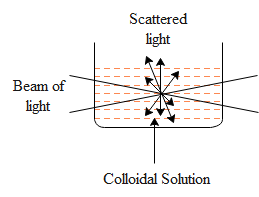
Note: (a) Selectivity and activity are the two main features of catalyst. The ability of a catalyst to increase the rate of reaction is known as the activity. One should note that a particular substance acts as a catalyst in certain reactions and may fail to catalyse in other reactions.
(b) There are various methods of coagulation.
- By electrophoresis: particles of dispersed phase move towards the oppositely charged electrode lose there to charge and get coagulated.
- By heating or cooling: In certain cases, the sol gets coagulated on heating. For example, on cooling milk fats start floating on the surface.
(c) Tyndall effect is used to distinguish between the true and colloidal solutions. If the path of light is illuminated, the solution is a colloidal solution. It may be noted that the among colloids lyophilic colloids also do not show Tyndall effect but lyophobic colloids show Tyndall effect
