Question
Question: (a) What is the molecular formula and structure of the alcohol which can be thought to be derived fr...
(a) What is the molecular formula and structure of the alcohol which can be thought to be derived from pentane?
(b) Write the names of the following functional groups
(i) −CHO
(ii) −OH
(iii) −COOH
(iv) −C=O
(v) −X
(c) What makes the candle flame yellow and luminous?
Solution
Hint : Alkane are the simplest hydrocarbons in which the carbon- to- carbon are linked through a single bond. They are also called saturated hydrocarbons. Functional groups are types of specific groups which have some characteristic properties. Generally, functional groups are responsible for the structural changes in an organic compound. You must remember the chemical formula of functional groups as the organic chemistry is dominated by the functional groups and the last hint is that incomplete combustion gives yellow flame.
Complete Step By Step Answer:
(a) Alcohol is an organic functional group which has at least one hydroxyl group i.e. (−OH) attached to a carbon atom. Alcohols are formed from alkanes. What happen is that one hydrogen atom i.e. (−H) is replaced by one hydroxyl group i.e. (−OH) . First, the alkanes are converted into alkyl halide through a free radical halogenation mechanism, then the alkyl halide is treated with strong bases like −NaOH and −KOH . Hence, alcohols are formed from alkanes. It does not change the number of carbon atoms but it only replaces one hydrogen group with one hydroxyl group.
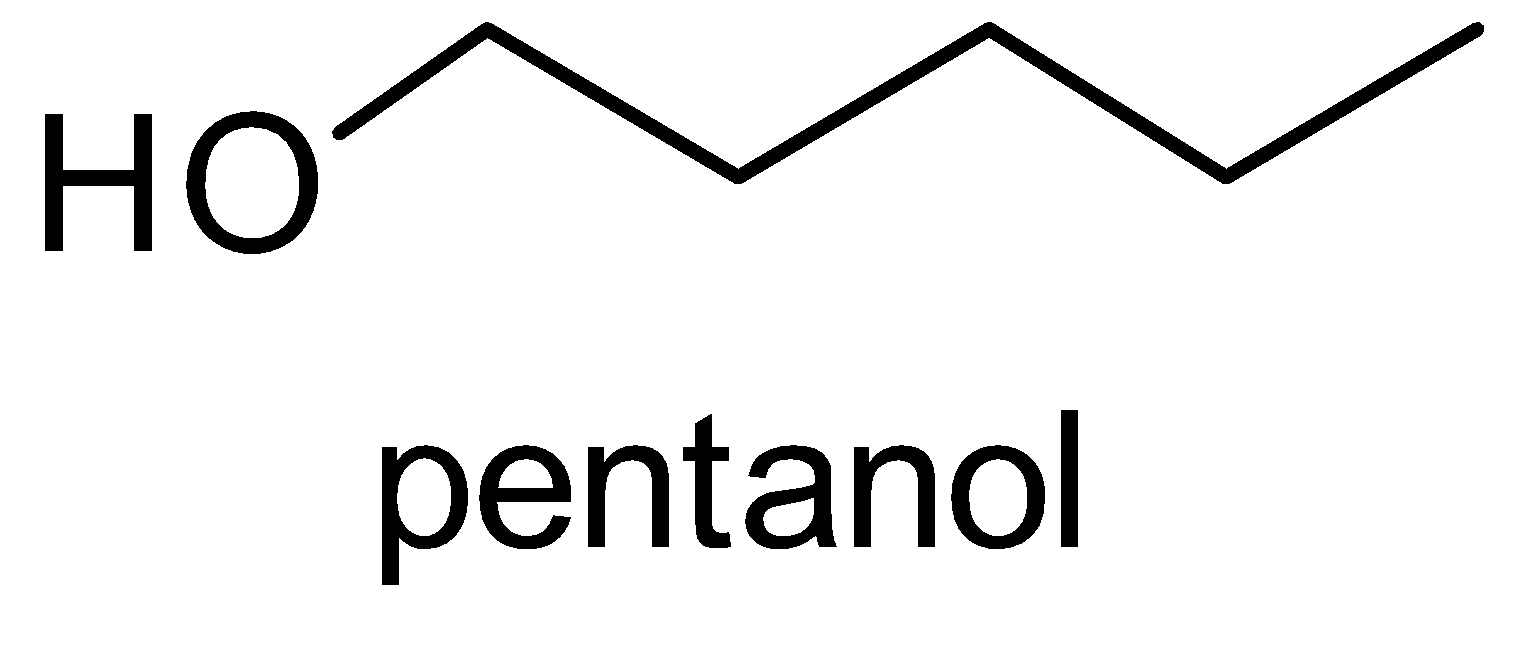
Pentane has a molecular formula C5H12 .So, the molecular formula of alcohol derived from pentane is C5H11OH or C5H12O and it is called as pentanol according to the IUPAC nomenclature.
Structure of pentanol-
(b) Functional groups are specific chemical species which are responsible for the reactivity of the organic compounds. They are the specific groups which have their own characteristic properties. There are some common functional groups which need to be remembered. It is important to recognize their physical and chemical properties.
(i) −CHO - Aldehyde group
(ii) −OH - Hydroxyl group
(iii) −COOH - Carboxylic group
(iv) −C=O - Ketone group
(v) −X - Halogen group
(c) When the candle is lighted, the candle burns with a yellow and luminous flame. This is so because when the candle is lit, the wax of the candle melts and it rises up the wick which gets converted into vapours. When the candle burns, there is no proper mixing of oxygen (or air) for burning wax vapours. So, the burning of wax takes place in an insufficient oxygen which leads to incomplete combustion of wax. Due to this incomplete combustion, the candle flame burns with a yellow and luminous flame.
Note :
It must be remembered that common functional groups such as alcohol, aldehyde, ketone, carboxylic acid and halogens as functional groups are the main domain of organic chemistry. In alkanes, the carbon-to- carbon single bond and in alkenes carbon-to- carbon double bond act as a functional group. Also, Yellow flame due to the incomplete combustion produces a lot of soot, carbon monoxide which is a health hazard.
