Question
Question: A vertical U-tube of uniform inner cross section contains mercury in both sides of its arms. A glyce...
A vertical U-tube of uniform inner cross section contains mercury in both sides of its arms. A glycerin (density = 1.3 g/cm3) column of length 10 cm is introduced into one of its arms. Oil of density 0.8 gm/cm3 is poured into the other arm until the upper surfaces of the oil and glycerin are in the same horizontal level. Find the length of the oil column, Density of mercury = 13.6 g/cm3
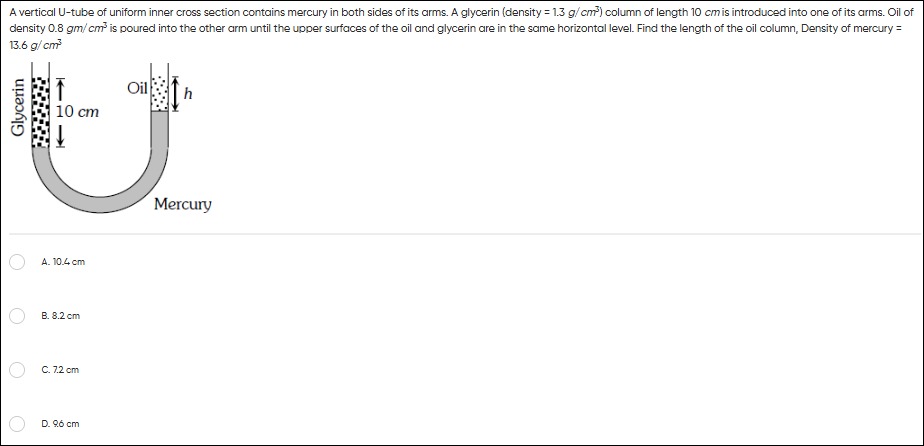
10.4 cm
8.2 cm
7.2 cm
9.6 cm
9.6 cm
Solution
The principle of fluid statics states that the pressure at the same horizontal level in a continuous fluid is equal. Let hg be the length of the glycerin column and ho be the length of the oil column. Let ρg, ρo, and ρm be the densities of glycerin, oil, and mercury, respectively. Given: hg=10 cm, ρg=1.3 g/cm3, ρo=0.8 g/cm3, ρm=13.6 g/cm3.
Consider a horizontal level at the interface between glycerin and mercury in the left arm. The pressure at this level is Pleft=Patm+ρgghg. In the right arm, at the same horizontal level, the pressure is Pright=Patm+ρmg(hmR−hmL)+ρogho, where hmR−hmL is the difference in mercury levels. Since the upper surfaces of oil and glycerin are at the same level, the difference in mercury levels is hmR−hmL=hg−ho. Equating pressures: ρghg=ρm(hg−ho)+ρoho. Rearranging for ho: ho(ρm−ρo)=hg(ρm−ρg). ho=hgρm−ρoρm−ρg=10 cm×13.6−0.813.6−1.3=10×12.812.3≈9.609 cm.
