Question
Question: A vertical cylinder is divided into two parts by a frictionless piston in the ratio 5:4. The piston ...
A vertical cylinder is divided into two parts by a frictionless piston in the ratio 5:4. The piston is free to slide along the length of the vessel and the length of the vessel is 90cm. Each of the two parts of the vessel contains 0⋅1mol of an ideal gas and the temperature of the gas is 300K. Find the mass of the piston.
A) 14kg
B) 12⋅7kg
C) 16kg
D) 15kg
Solution
The gas in the lower part of the cylinder will exert a force on the piston in the upward direction while the gas in the upper part of the cylinder exerts a force on the piston in the downward direction. Also, the weight of the piston will be directed downwards. However, at equilibrium, the net force acting on the piston will be zero.
Formulas used:
The force acting on a container filled with gas is given by, F=PA where P is the pressure exerted by the gas and A is the area of the container.
The ideal gas equation is given by, PV=nRT where P is the pressure, V is the volume, n is the number of moles present in the sample of gas, R is the gas constant and T is the temperature of the gas.
The volume of a container is given by, V=Al where A is the area of the container and l is its length.
Complete step by step answer:
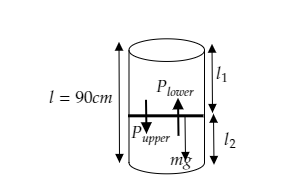
In the above figure, we see that the piston divides the length of the cylinder into a ratio of 5 : 4.
The total length of the vessel is given to be l=90cm .
Now, l1 and l2 are the lengths of the upper part and lower part respectively.
The number of moles present in each part is given to be n=0⋅1mol .
The temperature of the gas in each part is given to be T=300K .
In the figure, Plower is the pressure exerted by the gas in the lower part and Pupper is the pressure exerted by the gas in the upper part.
The weight of the piston will be W=mg.
It is given that l2l1=45⇒l1=45l2 .
Also, we have l1+l2=l=90cm
Then substituting for l1=45l2 in the above relation we get, 45l2+l2=90cm
⇒49l2=90 or l2=94×90=40cm
Then l1=45×40=50cm .
Thus the lengths of the upper part and lower part are respectively, l1=50cm and l2=40cm .
The ideal gas equation is given by, PV=nRT where P is the pressure, V is the volume, n is the number of moles present in the sample of gas, R is the gas constant and T is the temperature of the gas.
Then from the ideal gas equation, the pressure in the lower part can be expressed as Plower=V2nRT=A2l2nRT ------- (1)
Similarly, the pressure in the upper part can be expressed as Pupper=V1nRT=A1l1nRT ------- (2)
The net force acting on the piston at equilibrium can be expressed as F1+W=F2 ------ (3)
where F1 is the force on the piston due to the upper part, F2 is the force on the piston due to the lower part and W is the weight of the piston.
Then substituting for F1=PupperA1 , F2=PlowerA2 and W=mg in equation (3) we get, PupperA1+mg=PlowerA2 --------- (4)
Now substituting equations (1) and (2) in (4) we get, A2l2nRTA2+mg=A1l1nRTA1
⇒m=gnRT(l11−l21) ------------- (5)
Substituting for n=0⋅1mol , T=300K , l1=50cm , l2=40cm , g=9⋅8ms−2 and R=8⋅31JK−1mol−1 in equation (5) we get, m=9⋅80⋅1×8⋅31×300(0⋅51−0⋅41)=12⋅7kg
Therefore, the mass of the piston is m=12⋅7kg. So the correct option is B.
Note:
At equilibrium, as the net force acting on the piston is zero, the piston will not move. While substituting values in any equation make sure that the physical quantities are expressed in their respective S.I. units. If not, then the necessary conversion of units must be done. Here the lengths of the two parts are converted to meters during their substitution in equation (5).
