Question
Question: (a) Using Bohr’s postulates obtain the expression for total energy of the electron in the \({n^{th}}...
(a) Using Bohr’s postulates obtain the expression for total energy of the electron in the nth orbit of a hydrogen atom.
(b) What is the significance of negative sign in the expression for energy?
(c) Draw the energy level diagram showing how the line of spectra corresponding to Paschen series occur due to transition between energy levels
Solution
To find the total energy we will be using the postulates given by Bohr according to the need to find relationship between certain values so as to obtain the final result.
Total energy is equal to the sum of kinetic and potential energy and
Kinetic energy= 21mv2
The hydrogen spectrum shows the series after the electron transfers and there are fixed values of final energy levels for respective series formed.
Complete step by step answer: An electron is revolving around the nucleus of hydrogen atom can be represented diagrammatically as:
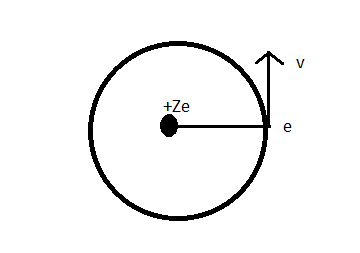
Let mass of electron = e [instead of taking value]
And mass of nucleus = Ze [Z = atomic number]
(a) According to Bohr’s postulate:
The centripetal force is provided to an electron revolving around the nucleus (positively charged) by the force of attraction between the nucleus and the electron.
The respective forces are given as:
Centripetal force = rmv2
Force of attraction = r2K(Ze)e where K = 4πε01
Then,
rmv2= 4πε01r2(Ze)e ____________ (1)
mv2 = 4πε01rZe2
Now,
Kinetic energy (K.E)= 21mv2
Substituting the value of mv2 (calculated), we get:
K.E = 4πε012rZe2
Potential energy (P.E) is given as:
P.E = r2K(Ze)(−e)
The total energy (T.E) is given by the sum of kinetic and potential energy.
T.E = K.E + P.E
T.E = (4πε012rZe2)+(−4πε012rZe2)
T.E = −4πε012rZe2
For nth orbit:
T.En=−4πε012rnZe2 ____________ (2)
Bohr’s postulate for angular momentum (L) that states only the electrons with momentum L=2πnh are allowed.
In general angular momentum (L) is given as:
L = mvr
Equating the two, we get:
mvr = 2πnh
Finding the value of v:
v=2πmrnh
Substituting this value of v in (1), we get:
rm(2πmrnh)2=4πε01r2Ze2
Calculating r for nth orbit:
rn=πmZe2ε0h2n2
Substituting this in (2), we get:
T.En=−4π∈012(πmZe2ε0h2n2)Ze2
T.En=−8ε0mh2n2Z2e4
Therefore, using Bohr’s postulates the expression for total energy of the electron in the nth orbit of hydrogen atom is :
T.En=−8ε0mh2n2Z2e4 ___________ (3)
This can also be written as:
T.En=−n2Z2Rhc
Where,
R is called Rydberg constant and the value it takes is:
R=8ε0mh3ce4
Now, Z = 1 for Hydrogen atom [Atomic number of Hydrogen is 1]
Therefore,
T.En=−n2Rch __________ (4)
Thus the expression for total energy is nth orbit of hydrogen atom is :
T.En=−n2Z2Rhc or T.En=−n2Rch
(b) The negative sign obtained in the expression for total energy shows that electron is bounded to the nucleus by the force of attraction.
(c) In line spectra (of hydrogen), the different series are obtained when electrons jump from higher excited state (ni) to ground state (nf)
Lymann series 🡪 nf = 1
Balmer series 🡪 nf = 2
Paschen series 🡪 nf = 3
Brackett series 🡪 nf = 4
Pfund series 🡪 nf = 5
For Paschen series (nf = 3), the spectra is given as:
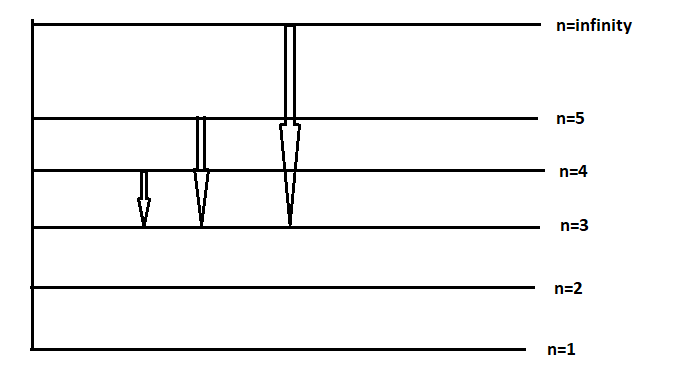
So during the transition the final energy level for electrons in the paschen series is n = 3.
Note: When we calculate potential energy, we take signs into consideration as well. (As we took negative for electron while calculating P.E)
Paschen series is the near to infrared
The change in energy (ΔE)can also be calculated as:
ΔE=8ε20h2me4(n2f1−n2i1)
