Question
Question: A U-tube of base length ‘\[l\]’ is filled with the same volume of two liquids of densities \[\rho \]...
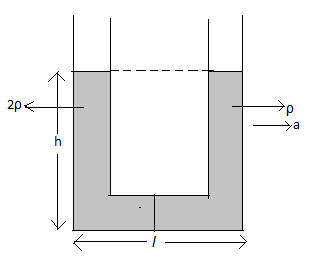
A U-tube of base length ‘l’ is filled with the same volume of two liquids of densities ρ and 2ρ is moving with an acceleration ‘a’ on the horizontal plane. If the height difference between the two surfaces (open to atmosphere) becomes zero then the height h is given by
A. 2gal
B. 2g3al
C. gal
D. 3g2al
Solution
Use the formula of pressure-depth relation. This formula gives the relation between the atmospheric pressure, density of liquid, acceleration of liquid and depth.
Formula used:
The pressure P at a depth h is given by
P=P0+ρgh …… (1)
Here, P0 is the atmospheric pressure, ρ is the density and g is the acceleration due to gravity.
Complete step by step answer:
A U-tube of base length ‘l’ is filled with the same volume of two liquids of densities ρ and 2ρ which are moving with an acceleration ‘a’ on the horizontal plane.
Redraw the diagram of the U-tube.
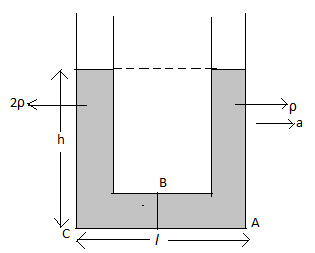
The liquid with the density 2ρ must be moving slowly as compared to the liquid with the density ρ.
The liquids have the acceleration a only in the horizontal plane and not in the vertical plane.
Using equation (1), determine the pressure PA at point A considering the right column of the U-tube.
PA=P0+ρgh
Using equation (1), determine the pressure PB at point B from point A considering the right column of the U-tube.
PB=PA+ρa2l
Substitute P0+ρgh for PA in the above equation.
PB=P0+ρgh+ρa2l
Using equation (1), determine the pressure PC at point C from point B considering the right column of the U-tube.
PC=PB+2ρa2l
Substitute P0+ρgh+ρa2l for PB in the above equation.
PC=P0+ρgh+ρa2l+2ρa2l
PC=P0+ρgh+23ρal …… (2)
Using equation (1), determine the pressure PC at point C considering the left column of the U-tube.
PC=P0+2ρgh …… (3)
The height difference between the two surfaces of two liquids open to the atmosphere becomes zero. Hence, the pressure at point C from both right and left liquid columns are the same.
Equate equation (2) and (3).
P0+ρgh+23ρal=P0+2ρgh
⇒23ρal=ρgh
Rearrange the above equation for height h.
h=ρg23ρal
⇒h=2g3al
Therefore, the height h is 2g3al.
So, the correct answer is “Option B”.
Note:
The pressure-depth relation is not the same for the pressure at the height h.
The height difference between the two surfaces (open to atmosphere) becomes zero.
The average density of a substance or object is defined as its mass per unit volume.
