Question
Question: A total pressure of \[3\] atm when applied on the surface of solution of concentration \[0.11\] M pr...
A total pressure of 3 atm when applied on the surface of solution of concentration 0.11 M prevents flow of solvent across the semipermeable membrane from the other side of solution of concentration of 0.01 M as shown in the diagram.
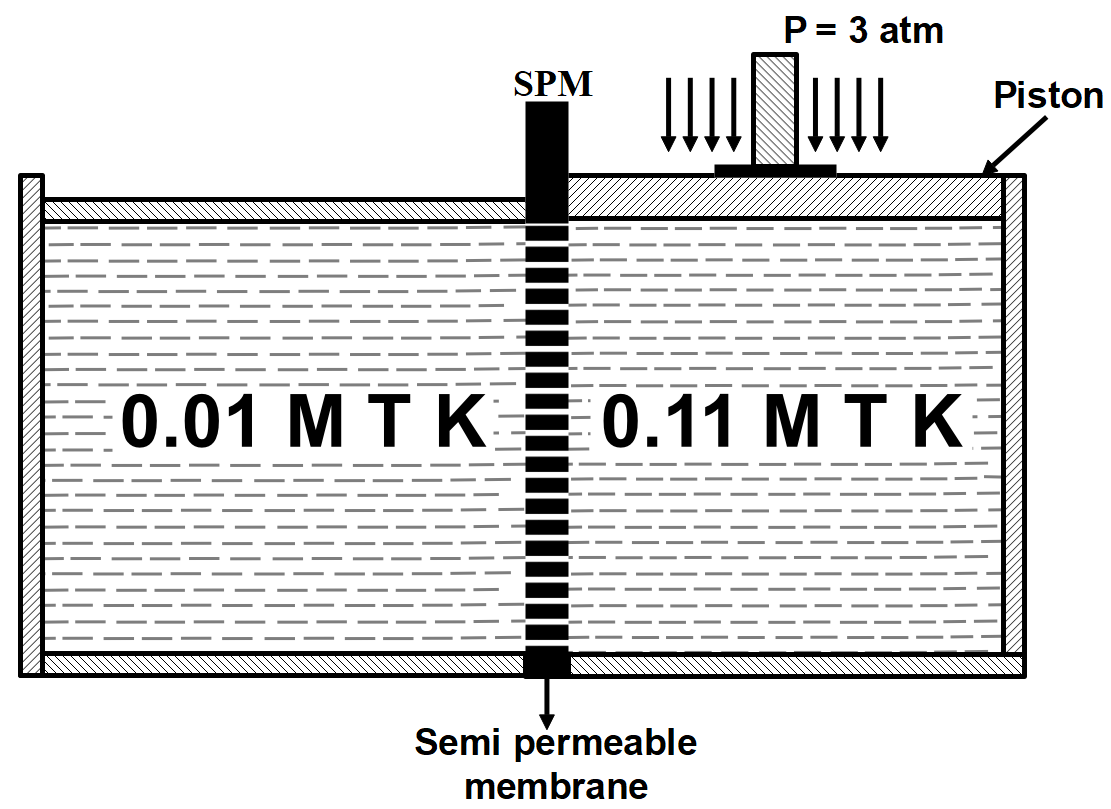
What is the experimental temperature? (Take: R=0.08 L−atm/K−mole )
A.375K
B.250K
C.125K
D.225K
Solution
We know that the osmotic pressure is the minimum pressure needed to stop the flow or osmosis of solvent molecules through the semipermeable membrane. On adding non-volatile solute, the osmotic pressure is directly proportional to the molar concentration of solute and directly proportional to the absolute temperature of the solution. The formula we are going to use here is π=ΔcRT
Complete answer:
As we already know, Osmotic pressure is the external pressure that when applied to the solution to prevent the solvent from moving from a higher concentration area to a lower concentration area. It is denoted so that it can be distinguished from the atmospheric pressure. The minimum amount of pressure required to stop the process of osmosis is called osmotic pressure. As osmotic pressure is one type of pressure the unit of this will be as the unit of atmospheric pressure. The molal depression constant can be defined as when the molality of the solution is one the amount of freezing point depression is equal to the molal depression constant. The value of the molal depression constant is different for different solvents.
Osmotic pressure is a colligative property just like elevation in boiling point and depression in freezing point. Its uses areIt is the best way to the molecular mass of the solute. It is used to calculate the molecular mass of biomolecules and polymers with high molecular masses at room temperature as they are unstable at high temperatures. It is also used in the desalination of seawater to make the seawater drinkable. Here on substitution we get; π=ΔcRT
⇒3=(0.1−0.01)×0.08×T
Thus, T=0.1×0.083×10×100=125×3=375K.
Therefore, the correct answer is option A.
Note:
Remember that the freezing point depression due to the solute-solvent interactions. It is a colligative property. The amount of change in the freezing point is related to the number of particles of solute in a solution and does not depend on the chemical composition of the solute.
