Question
Question: A tin chloride Q undergoes the subsequent reaction (not balanced) \(Q+C{{l}^{-}}\text{ }\to \text{...
A tin chloride Q undergoes the subsequent reaction (not balanced)
Q+Cl− → X
Q+ Me3N→ Y
Q+ CuCl2 → Z+ CuCl
X is a monoanion having pyramidal geometry. Both Y and Z are neutral compounds. Choose the right options (this question has multiple correct option)
(A) The central atom in X is sp3 hybridized
(B) There is a dative bond in Y
(C) The oxidation number of the central atom in Z is +2
(D) The central atom in Z has one lone pair of electrons
Solution
As we all know the formula for tin chloride is SnCl2 (where during this question performs some reactions). Monoanion is mentioned as an ion having one charge. Where pyramidal geometry is made by a polyhedron formed by connecting a polygonal base and some extent, called the apex.
Complete step by step answer: In the unified state, the metals are meant to exist as they are present in nature within their compounds. The reactive metals typically exist inside their compound appearance. In the cumulative state the metals are found as oxides, carbonates, sulphides, silicates, phosphates etc. within the planet's crust. Composite Q is SnCl2.
The given reactions are
SnCl2+Cl−→(X)SnCl3−
SnCl2+Me3N→(Y)Me3N+−Sn−Cl2
SnCl2+2CuCl2→SnCl4+(Z)2CuCl
Hybridisation of Sn in SnCl3−is sp3(X)
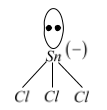
Trimethylamine forms a co-ordinate bond with Sn in SnCl2(Y),
and in SnCl4 there's no lone pair on the central atom of SnCl4 (Z).
Hence, (Q), (X), (Y) and (Z) are SnCl2, SnCl3−, SnCl2 and SnCl4 respectively.
Tin (II) chloride is a white crystalline solid with the formula SnCl2 and is also known as stannous chloride. It forms a stable dihydrate, but aqueous solutions, especially if acidic, tend to undergo hydrolysis. SnCl2 is commonly used as a reducing agent, and for tin-plating in electrolytic baths.
Note: Tin (Sn) may well be a bunch 14 elements, it show sp3 hybridisation with two bond pairs between sp3−p orbital of two chlorine atoms and remaining two lone pairs are present. the shape of the molecule is V-shaped.
