Question
Question: A thermodynamic system is taken through the cycle \[ABCD\] as shown in the figure. Heat rejected by ...
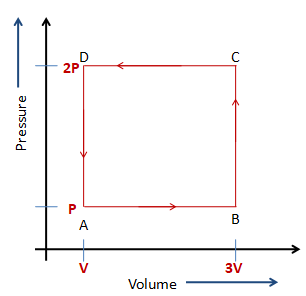
A thermodynamic system is taken through the cycle ABCD as shown in the figure. Heat rejected by the gas during the cycle is,
(A)PV
(B)2PV
(C)4PV
(D)\raise.5ex1
Solution
Find the work done for the whole process using the given pressure and volumes of each point on the graph. Note that the amount of heat rejected from the gas during the cycle.
Formula used:
The work done by gas, W=P(V2−V1)
P is the pressure of a particular point and (V2−V1) is the change in volume of the gas for that point.
The heat is rejected by the gas Q=W.
Complete step by step answer:
The following figure shows the cyclic process of gas.
If an object returns to its initial position after one or more processes it went through.
ABCDA is the cycle.
The pressure at the points both D and C remain the same, which is 2P. The volume at the point D is V and at the point C is 3V,
So, the work done by the gas from point D to point C , WDC=2P(3V−V)=4PV
The pressures at the points C and Bare 2P and P respectively. The volume at the points both C and B remain the same, which is 3V.
So, the work done by the gas from point C to point B , WCB=P(3V−3V)=0
The pressure at the points both B and A remain the same, which is P. The volume at the point B is 3V and at the point A is V,
So, the work done by the gas from point B to point A , WBA=P(V−3V)=−2PV
The pressures at the points A and D are P and 2P respectively. The volume at the points both A and D remain the same, which is V.
So, the work done by the gas from point A to the point D , WAD=P(V−V)=0
Hence the total work done in the whole cycle, W=4PV−2PV=2PV
We know the heat rejected from the cycle is equal to the amount of total work done by the gas, so Q=W
⇒Q=2PV
Hence, the correct answer is option (B).
Note: In a cyclic process, the total work done can be defined as the area of the portion that is covered by the cycle.
For the given diagram the cycle is ABCDA
So the work done will be the area of the rectangle ABCD, i.e W=AB×BC
The value will be, W=(3V−V)×(2P−P)=2PV.
