Question
Question: A \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}\) did not give a silver mirror with Tol...
A C3H6O did not give a silver mirror with Tollen's reagent but gave an oxime with hydroxylamine. It can give positive:
A.Iodoform test
B.Fehling's test
C.Schiff’s test
D.Carbylamine test
Solution
To answer this question, you should recall the concept that silver mirror Test with Tollen’s reagent is given only by aldehydes but not by ketones. Now, use the concept that ketones form oxime in hydroxylation reaction and use it to apply on the aforementioned options systematically.
Complete step-by-step answer:
We know from the basics of organic chemistry:
1. Silver Mirror Test with Tollen’s reagent is given by aldehydes but not by ketones. Thus, the given organic compound cannot be an aldehyde.
2. The compound gives an oxime with hydroxylamine. Thus, given compound is a ketone
Now using the above facts to eliminate the options systematically:
A. The Iodoform Test is given by the methyl keto group. Thus, this option is correct. The general haloform reaction of methyl ketones can be represented as:
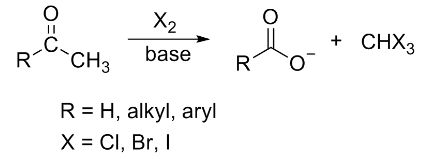
B. Fehling’s Test is given by an aldehyde. As the given compound cannot be an aldehyde through 1. Thus, this option is wrong and can be eliminated.
C. Schiff’s Test is also given by aldehydes. As the given compound cannot be an aldehyde through 1. Thus, this option is wrong and can be eliminated
D. Carbylamine test is given by primary amines and is used as a distinguishing test between primary amines from other amines. The given compound cannot be primary amine because primary amines do not form oxime with hydroxylamine. Thus, the given option is wrong and can be eliminated.
Therefore, we can conclude that the correct answer is (A) Iodoform test and the unknown compound is ketone having methyl keto group.
Note: We should know that αhydroxy ketones are an exception to the silver mirror test. Despite being a ketone, it shows positive test because of tautomerization, where the ketone becomes en-ol, and then another tautomerization happens with the original hydroxy group (which is terminal), making it an aldehyde group. All these forms exist in equilibrium, so it responds to Tollen’s test.
