Question
Question: A: Tetracyanomethane B: Carbon dioxide C: Benzene D: 1,3-buta-di-ene Ratio of \(\sigma \) an...
A: Tetracyanomethane
B: Carbon dioxide
C: Benzene
D: 1,3-buta-di-ene
Ratio of σ and π:
(A) A and B are equal
(B) C and D are equal
(C) same in A, B, C and D
(D) B and D are equal
Solution
One single bond has only one sigma bond (σ) and no pi bond (π). One double bond contributes to one sigma bond (σ) and one pi-bond (π). One triple bond has one sigma bond (σ) and two pi-bond (π). Describe the structure and find the number of single, double and triple bonds and calculate the ratios.
Complete step by step solution:
Let us draw the structures of the four structures given in the question:
| S. No. | Name of the compounds | Structure of the compounds | Explanation of the bonds and formation of the structure | Number of sigma bonds (σ) | Number of pi-bonds (π) | Ratio of σ and π |
|---|---|---|---|---|---|---|
| 1. | Tetracyanomethane | 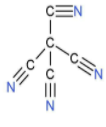 | The compound has four cyano groups. A carbon atom is attached to the carbon atom of a cyano group by a single bond. There is a triple bond between nitrogen and carbon atoms in the cyano group. So, there are four triple bonds. | (1×4)+(1×4), which is equal to 8. | (2×4), which is equal to 8. | 88 or 1. |
| 2. | Carbon dioxide | 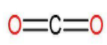 | The compound has a single carbon atom which forms a double bond with two oxygen atoms. | (1×2) or 2 | (1×2) or 2 | 22 or 1 |
| 3. | Benzene | 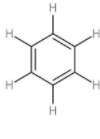 | The compound has six carbon atoms which form an alternative double bond with each other. Every carbon atom is attached to a hydrogen atom with a single bond. The chemical formula is C6H6. | (1×6)+(1×6) or 12 | (1×3) or 3 | 312 or 4 |
| 4. | 1,3-buta-di-ene | 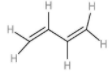 | The compound has an alternative double bond. The total number of hydrogen atoms is 6. | (1×6)+(1×3)or 9 | (1×2) or 2 | 29 or 4.5 |
The ratio of σ and π in A and B are equal, which is option (A).
Note: An element forms a sigma bond (σ) with every different element only once. That is if an element forms multiple bonds with another element, then, it means that there is one sigma bond (σ) and rest are pi-bonds. The compounds with single bonds are known as saturated and compounds with multiple bonds are unsaturated.
