Question
Physical Chemistry Question on Thermodynamics
A system undergoes one clockwise cycle from point X back to point X as shown in the figure below:
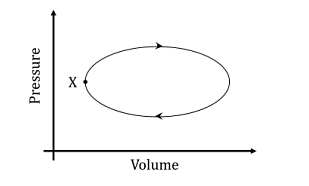
The correct statement about this process is
A
Internal energy of the system decreases at the end of the cycle
B
Entropy of the system increases at the end of the cycle
C
System performs work on the surroundings during the cycle.
D
Heat exchanged between system and surroundings is zero during the cycle.
Answer
System performs work on the surroundings during the cycle.
Explanation
Solution
The correct option is (C): System performs work on the surroundings during the cycle.
