Question
Question: A student added a few pieces of aluminium metal to two test tubes A and B containing aqueous solutio...
A student added a few pieces of aluminium metal to two test tubes A and B containing aqueous solutions of iron sulphate and copper sulphate. In the second part of her experiment, she added iron metal to another test tube C and D containing aqueous solutions of aluminium sulphate and copper sulphate. In which test tube or test tubes will she observe the colour change? Based on this experiment, state which one is the most reactive metal and why?
Solution
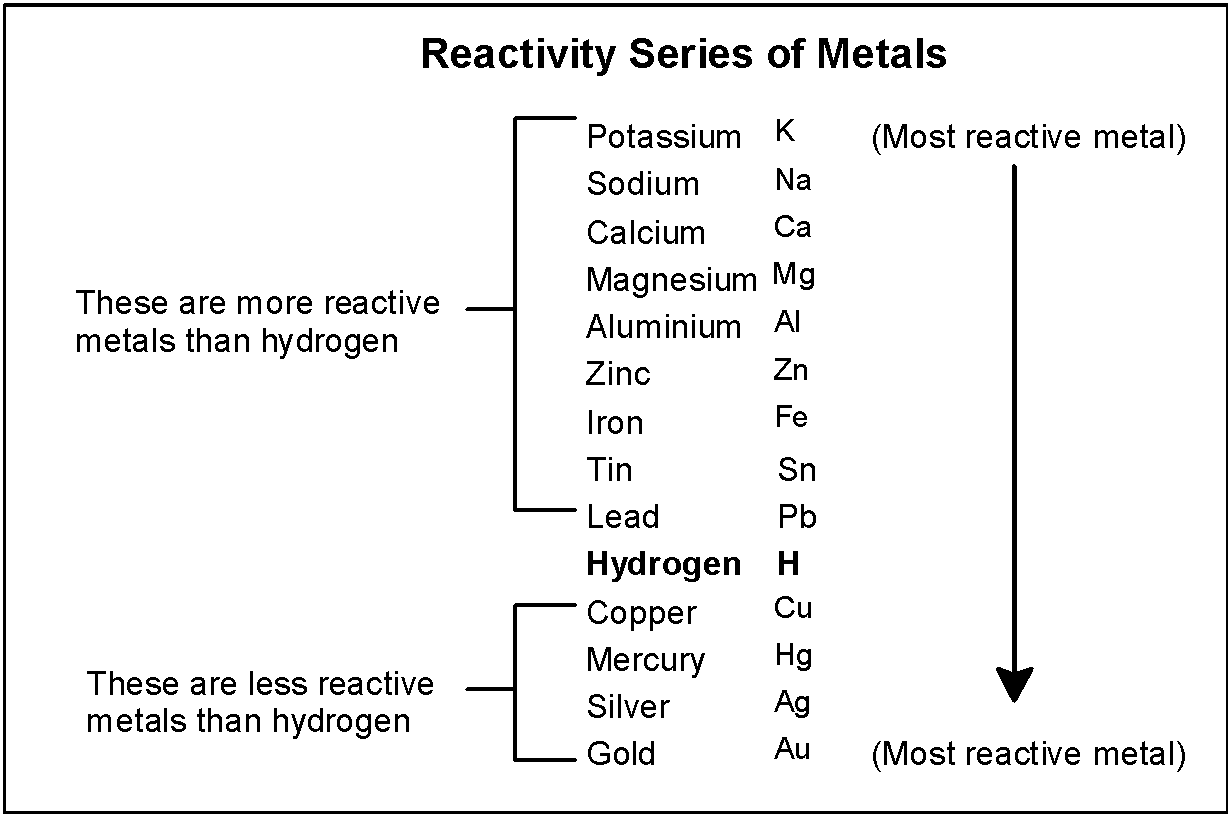
The reactivity of metal towards the other metal is determined on the basis of the electrochemical series. The metal which is at the higher end of the reactivity series displaces the lower reactivity metal. The reaction is also known as the single displacements reaction. The general depiction is as shown below:
M1n+ + M2 → M1 + M2m+
Where, M1 has lower activity and M2 has the high activity.
Complete Solution :
- The metal which is in the elemental state when placed in the solution of the metal salt of the metal than the more energetic ‘elemental metal ‘exists as an ion and the ‘ionic metal’ exist as an element .in other words, the elements present in the zero oxidation state oxides and the ion in the solution reduces to the zero oxidation state.
- The reaction of the replacement of one of the metal by the other metal in the solution is called the single displacement reaction.
- According to the reactivity series, the metal which has the highest position in the activity series will displace the other metal.
Let's have a look at the experiment.
Part A) the aluminium has a higher position in the reactivity series in comparison with iron and copper. The reaction of the single displacement between the aluminium with iron and copper is as depicted below,
CuSO4 + Al → Al2(SO4)3 + Cu FeSO4 + Al → Al2(SO4)3 + Fe
The aluminium sulphate is colourless, thus the colour of the is due to the free State metal in the solution.
Thus, both the A and B test tube exhibits the colours.
Part B) in this part of the experiment, the student added the pure iron metal to the aqueous solution of aluminium sulphate and copper sulphate .the iron has a higher reactivity as compared to the copper sulphate and less reactivity compare to the aluminium sulphate. Thus, iron can only displace the iron from the iron sulphate. The reactions are as follows,
CuSO4 + Fe → Fe(SO4)3 + Cu Al2(SO4)3 + Fe → No reaction
Thus, only the D test tube exhibits the colour change.
Hence, A, B, and D test tubes show the colour change in the solution.
Note: the electrochemical series is given as the follows: