Question
Question: A solution of \(\mathbf{2M}\) formic acid \((HCOOH)\) is \(0.95\%\) ionized. What is the \(\mathbf{K...
A solution of 2M formic acid (HCOOH) is 0.95% ionized. What is the Kα of formic acid?
A.1.9×10−2
B.1.8×10−4
C.9×10−5
D.4.5×10−5
Solution
The dissociation constant (Ka) is a quantitative measurement of the strength of acid in a solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions. The dissociation constant ‘Ka’ for a degree of dissociation (α) is given as.
Kα=α2C
Complete step by step answer:
We know that, the formula for degree of dissociation (α) in terms of dissociation constant Kα is,
Kα=α2C
∴ α=CKα
Squaring both the sides
∴ α2=CKα
Where α is degree of dissociation and C is the concentration
∴ Kα=α2C
Given degree of dissociation is
α=0.95 !!
And concentration is
C=2M
Therefore, on putting the above values in the formula we have derived above, we get
Kα=(1000.95)2×2
=1.84×10−4
So, B is the correct option.
Additional Information:
-Formic acid is also known as mechanic acid. The word ‘Formic’ is a Latin word used for ‘ant’, ‘’formica’’.
-It’s chemical formula is HCOOH.
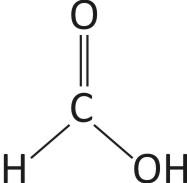
-Formic acid is the simplest carboxylic acid, which contains a single carbon atom.
-It occurs naturally in various sources like venom of bee and ant stings, pine needles.
-It is used to produce insecticides, used as a preservative also.
-It is a colourless liquid, highly flammable, and pungent.
Note:
As we know the formic acid is corrosive, it can cause acid is corrosive, it can cause burns to any part of the body which comes in contact with.
Any by mistake intake of formic acid can lead to burns of the mouth, throat and stomach.
While performing reactions of formic acid, do not breathe vapors and use a respirator.
