Question
Question: A soap bubble is formed from \[5\times {{10}^{-3}}g\] of soap solution. When filled with hydrogen of...
A soap bubble is formed from 5×10−3g of soap solution. When filled with hydrogen of density 0.0009g/cm3, it floats in air of density of 0.00129g/cm3. If surface tension of soap solution is 30dyne/cm, the excess pressure in dyne/cm2is:
(A) 30
(B) 60
(C) 120
(D) 240
Solution
The tricky part in questions where a lot of different data is given to us is to find out where to start solving from. We know the mass of the soap solution and it’s filled with hydrogen whose density has been given to us, so we can find the volume of our bubble and from the obtained volume, we can find the radius of the bubble and use the formula for excess pressure. We have also been given that our bubble floats in air, which means we’ll have to consider the buoyant force of air on the bubble. So let’s dive right in and start solving this bad boy.
Complete step by step answer:
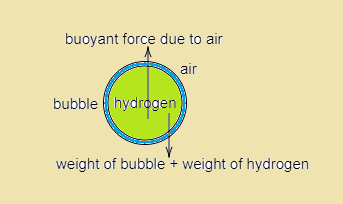
As we can see clearly the different forces acting on our bubble, we’ll form the balanced weight equation for our bubble,
Let the volume of our bubble be V cm3
Weight of bubble + weight of hydrogen = buoyant force due to air
