Question
Question: A slightly polar molecule \(AB\) has a dipole moment of \(0.24D\) . If bond length is \[1{A^ \circ }...
A slightly polar molecule AB has a dipole moment of 0.24D . If bond length is 1A∘ , ionic character is (%) :
Solution
Whether a compound is polar, covalent or ionic that can be determined by calculating the percentage ionic character, which is the ratio of a bond's measured dipole moment to the dipole moment assuming a complete electron transfer.
Formula Used: Percent Ionic Character =μaμo×100%
Here μo is the observed dipole moment and μa is the dipole moment calculated under the assumption that the compound is 100% ionic character.
Complete step by step answer:
As we all know that dipole moment μ of any compound is given by the formula
μ=q×r
Where q is the magnitude of charge and r is the distance between the centers of positive and negative charge
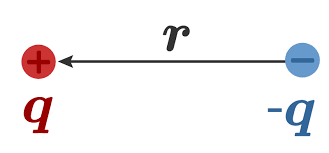
Now in the given question if we assume 100% Ionic character the magnitude of charge will be 1.602×10−19C that is the charge on a single electron since we are assuming transfer as complete ionic so magnitude of charge will be equal to the charge on one electron
Given the distance between both the centers as 1A∘or 1×10−10m
We can calculate μa as
μa=1.602×10−19×10−10Cm
μa=1.602×10−29Cm
Now to find Ionic Character (%) we need to convert the above dipole moment in a similar unit as that of the observed dipole moment that is Debye (D) .
1D=3.336×10−30Cm
So μa in Debye is given by 3.336×10−301.602×10−29D
μa=4.8021D
Also μo=0.24D (given in the question)
Now Percent Ionic Character =μaμo×100%
Here μo is the observed dipole moment and μa is the dipole moment calculated under the assumption that the compound is 100% ionic character.
Hence Percent Ionic Character =4.80210.24×100%
Answer =5%
Note: Whenever taking the ratio for the calculation of Percent Ionic Character always remember taking proper units into consideration such that the ratio is always dimensionless
