Question
Question: a. Show the formation of \[NaCl\] from sodium and chlorine by the transfer of electrons. b. Why ha...
a. Show the formation of NaCl from sodium and chlorine by the transfer of electrons.
b. Why has sodium chloride a high melting point?
c. Name the anode and the cathode used in the electrolytic refining of impure copper metal.
Solution
Formation of any compound can be shown by using the electronic configurations of the electrons respectively. The configuration of sodium is 1s22s22p63s1 and chlorine is 1s22s22p63s23p5. Remember that sodium chloride is an ionic compound and it has a strong force of attraction between them. An anode is impure and a cathode is pure copper metal in electrolytic refining.
Complete answer:
(a) The electronic configuration of sodium is 1s22s22p63s1 and the electronic configuration of Chlorine is 1s22s22p63s23p5. By looking at the electronic configuration we can say that the number of electrons in the outermost shell of Sodium is 1 and Chlorine is 7. Hence the electrons will move from sodium to chlorine. Sodium will transfer its one electron from its outermost shell to chlorine and will become Na+ to attain stable electronic configuration and chlorine will accept one electron and becomes Cl− to attain stable electronic configuration. Thus the formation of NaCl from sodium and chlorine atoms by the transfer of electron will be as follows:
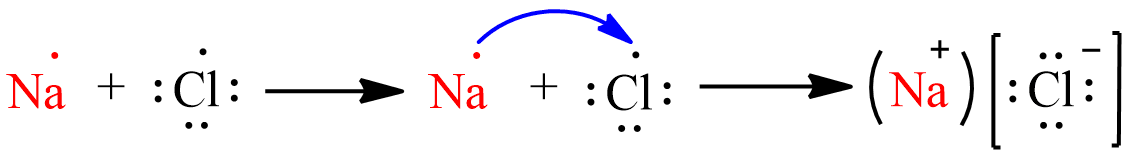
(b). Sodium chloride has a high melting point because it is an ionic compound. Ionic compounds are made up of electrically charged species called ions that can be positive (cation) or negative (anions). The force of attraction between the ions is very strong and is difficult to break. Hence, it requires a high amount of energy to break the bonds.
(c) During the electrolytic refining of copper:
Anode: Thick block of impure copper metal
Cathode: Thin strip of pure copper metal.
Note:
In the electrolytic refining of impure copper metal, the cathode is coated with graphite in the process of electrolytic metal processing or merely electro grinding so that the concentrated material can be easily removed.
This is one of the most growing electrolysis procedures. To show the formation of NaCl from sodium and chlorine it is important to know their electronic configurations and which atom will transfer its electrons.
