Question
Question: A sample of ideal gas is taken through the cyclic process shown in the figure. The temperature of th...
A sample of ideal gas is taken through the cyclic process shown in the figure. The temperature of the gas at state A is TA=200K. At states B and C the temperature of the gas is the same.
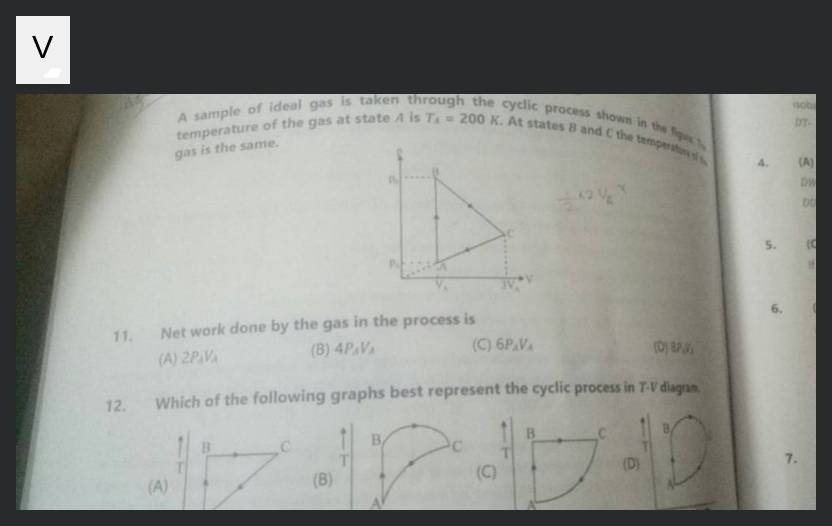
2PAVA
4PAVA
6PAVA
8PAVA
2PAVA
Solution
The net work done by the gas in a cyclic process is the area enclosed by the cycle in the P-V diagram. The process ABC is a triangle. The net work done is the area of this triangle. Area =21×base×height. From the P-V diagram, state A is at (VA,PA), state B is at (VA,P0), and state C is at (3VA,P0). The base of the triangle can be taken along the isobaric line BC, with length VC−VB=3VA−VA=2VA. The height of the triangle is the difference in pressure P0−PA. Therefore, the net work done Wnet=21×(2VA)×(P0−PA)=VA(P0−PA).
The problem states that TB=TC. Using the ideal gas law PV=nRT, we have TBPBVB=TCPCVC. Since TB=TC, we get PBVB=PCVC. From the diagram, VB=VA, PB=P0, VC=3VA, PC=P0. Substituting these values, we get P0VA=P0(3VA), which implies VA=3VA, leading to VA=0 (or P0=0). This indicates an inconsistency in the problem statement or the diagram.
However, if we assume that the intended relationship between pressures and volumes, along with the options provided, implies a specific ratio, we can work backwards. If we assume P0=3PA, then the net work done becomes: Wnet=VA(3PA−PA)=VA(2PA)=2PAVA. This matches option (A). Let's check if this assumption is consistent with the condition TB=TC. If P0=3PA, then TB=nRP0VA=nR3PAVA and TC=nRP0(3VA)=nR3PA(3VA)=nR9PAVA. For TB=TC, we would need 3PAVA=9PAVA, which implies PA=0 or VA=0. This confirms the inconsistency.
Despite the inconsistency, given the multiple-choice options, the most plausible intended answer is derived by assuming a relationship that leads to one of the options. The assumption P0=3PA yields Wnet=2PAVA.
The condition TA=200K is not used for calculating the net work done, which is independent of temperature for a given cycle in the P-V diagram.
