Question
Question: A sample of gas expands from volume \({V_1}\) to \({V_2}\) . The amount of work done by the gas is g...
A sample of gas expands from volume V1 to V2 . The amount of work done by the gas is greatest when the expansion is:
(A) Isothermal
(B) Isobaric
(C) Adiabatic
(D) Equal in all cases
Solution
Hint To solve the question, the knowledge that you must have is about what these terms means, their graphs, and the fact that work done by the gas is nothing but the area under the pressure vs. volume graph in the cases given. The more the area covered under the graph means the more work done by the gas and vice versa.
Complete step by step answer
As explained in the hint section, we first and foremost need to know the meaning of the three terms given as the option. Let us start by explaining the term isothermal expansion:
Isothermal expansion is the kind of expansion of gas when the temperature at every step of the expansion stays the same as it was in the previous step. In other words, it is a kind of expansion where the gas expands but the temperature remains constant throughout the process.
Now, if we try to explain the term isobaric expansion, this can be described as the process of expansion of a gas in which the amount of pressure always stays the same as it was in the previous step. In simpler words, the pressure remains constant throughout the whole process of expansion of the gas.
As for the adiabatic expansion of gas, it is the type of expansion in which there is no exchange of heat or mass between the surrounding and the system, yet the amount of both remains the same in the universe. In such a process, no amount of heat and mass is given out or taken in by the system.
If we try to draw the graph of pressure vs. volume for all the three process, it can be drawn as: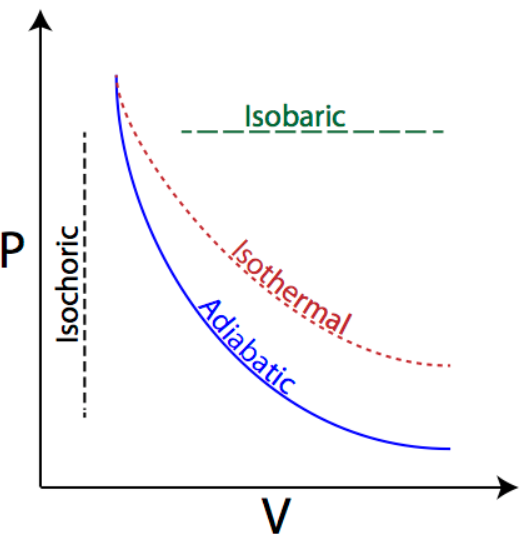
As explained in the hint section, the work done by a gas in expansion processes is nothing but the area under the pressure vs. volume curve of the gas throughout the process of expansion. As we can see in the diagram, the maximum area is covered by the curve in which the value of pressure remains constant throughout the process. Hence, the maximum work done is also in the process in which the pressure remains constant, i.e. Isobaric process.
So, the correct option is nothing but option (B), Isobaric process.
Note The major mistake that students make is believing that the work done is basically related to displacement or change in state and should not be affected by the path taken, which causes them to tick option (D) as the correct one which is completely wrong since work does depend upon the path taken in real life situations and sometimes, in ideal conditions too.
