Question
Question: A ring shaped tube contains two ideal gases with equal masses and molar masses \[{{M}_{1}}\] =32 and...
A ring shaped tube contains two ideal gases with equal masses and molar masses M1 =32 and M2=28. The gases are separated by one fixed partition P and another movable stopper S which can move freely without friction inside the ring. The angle α in degree is
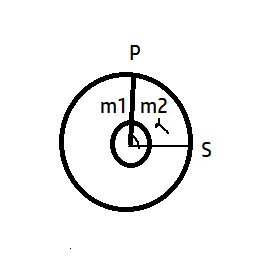
(A) 182
(B) 170
(C) 192
(D) 180
Solution
In this problem it is mentioned that masses of the gas in the ring are the same. Also, they are separated by fixed portions. Thus, the pressure exerted must be the same by both the gases.
Complete step by step answer:
Pressure exerted by gas A= pressure exerted by gas B
Using ideal gas law, PV=nRT
P=VnRT
So P1=P2
V1n1RT=V2n2RThere temperature of the both of them are same and R is a gas constant.
Let us find out the number of moles, it is given by the formula. n=Mm
Here m is the given mass and M is the molecular mass of the gas. It is given that the given mass for both of the gas are the same.
V1n1=V2n2
⇒V132=V228
⇒V1V2=78------(1)
It is clear from the figure that total angle is 3600 and gas 2 makes an angle α. Since radius for both is same, thus,
V1V2=360−αα
Comparing it with eq (1) we get,
