Question
Question: A reactant (1) forms two products : 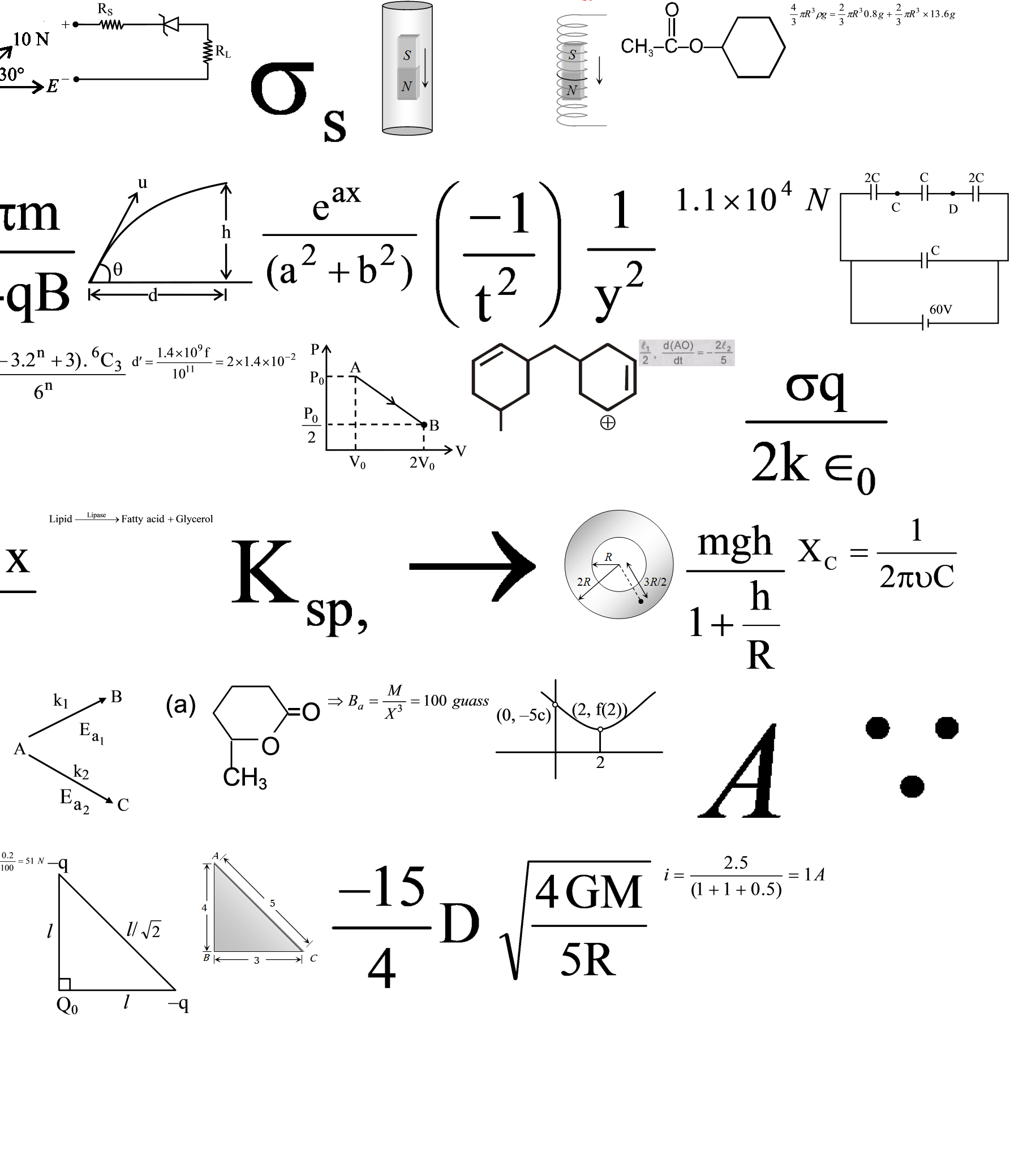 forms two products :

Ea2= 2Ea1
Frequency factors for both the reaction are equal.
Therefore-
A
k₂ = k₁ e^{- Eₐ₁/RT}
B
k₂ = k₁²/A
C
k₁ = A k₂ e^{Eₐ₁/RT}
D
Both (1)&(2) are correct
Answer
Both (1)&(2) are correct
Explanation
Solution
k2 = A e−Ea2/RT ; k1 = A e−Ea1/RT
ln k2 = ln A – RTEa2 & ln k1 = ln A –RTEa1
or Ea2= RTln A/k2 & Ea1 = RT ln k1A
\ 2RT ln k1A = RT lnk2A
\ ln (k1A)2 = ln k2A
\ (k1A)2 = k2A
or k12A2 = k2A
\ k2 = Ak12
And k2 = Ae−Ea2/RT
k1 = Ae−Ea1/RT
k1k2 = eEa1−Ea2/RT= e−Ea1/RT
or k2 = k1 e−Ea1/RT
