Question
Question: A radioactive nucleus A can decay to P or Q with decay constant $2\lambda$ and $\lambda$ respectivel...
A radioactive nucleus A can decay to P or Q with decay constant 2λ and λ respectively. Another radioactive nucleus B decays in Q with decay constant 3λ. Initially number of active nuclei of A and that of B are equal (N0). Select correct relation for any later moment t.
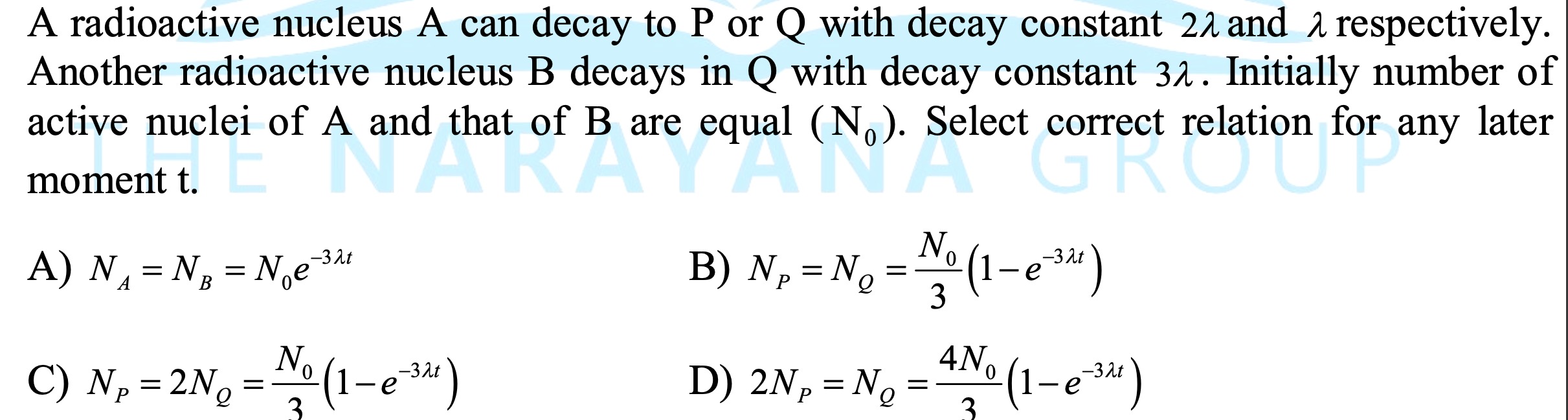
A
NA=NB=N0e−3λt
B
NP=NQ=3N0(1−e−3λt)
C
NP=2NQ=3N0(1−e−3λt)
D
2NP=NQ=34N0(1−e−3λt)
Answer
Option D
Explanation
Solution
- For nucleus A, the total decay constant is 2λ + λ = 3λ, so the number remaining is
NA=N0e−3λt. - In its decay, P is produced with probability 2/3 and Q with probability 1/3. Thus,
NP=32[N0−NA]=32N0(1−e−3λt). Q from A =31[N0−NA]=3N0(1−e−3λt). - Nucleus B decays to Q with decay constant 3λ, so
NB=N0e−3λtandQ from B =N0−NB=N0(1−e−3λt). - Total Q produced is:
NQ=3N0(1−e−3λt)+N0(1−e−3λt)=34N0(1−e−3λt). - Notice that
2NP=2×32N0(1−e−3λt)=34N0(1−e−3λt)=NQ.
Thus, the relation is 2NP=NQ=34N0(1−e−3λt).
