Question
Question: A polymer contains three monomers: acrylonitrile, butadiene and styrene. Predict the structure of th...
A polymer contains three monomers: acrylonitrile, butadiene and styrene. Predict the structure of this ABS plastic.
Solution
Hint Smallest repeating unit of a polymer is called a monomer. Polymer is a longest chain made up of more than 10000 monomer units. In a simple way we can say that monomers are the building blocks of polymers.
Complete step by step answer:
- In the question it is given that there are three monomers acrylonitrile, butadiene and styrene. If these monomers undergo polymerization a polymer is going to form.
- We have to predict the structure of the polymer which is going to form by using the given monomer units.
- Before going to predict the structure of the ABS plastic we should know the structures of the individual monomers and it is as follows.
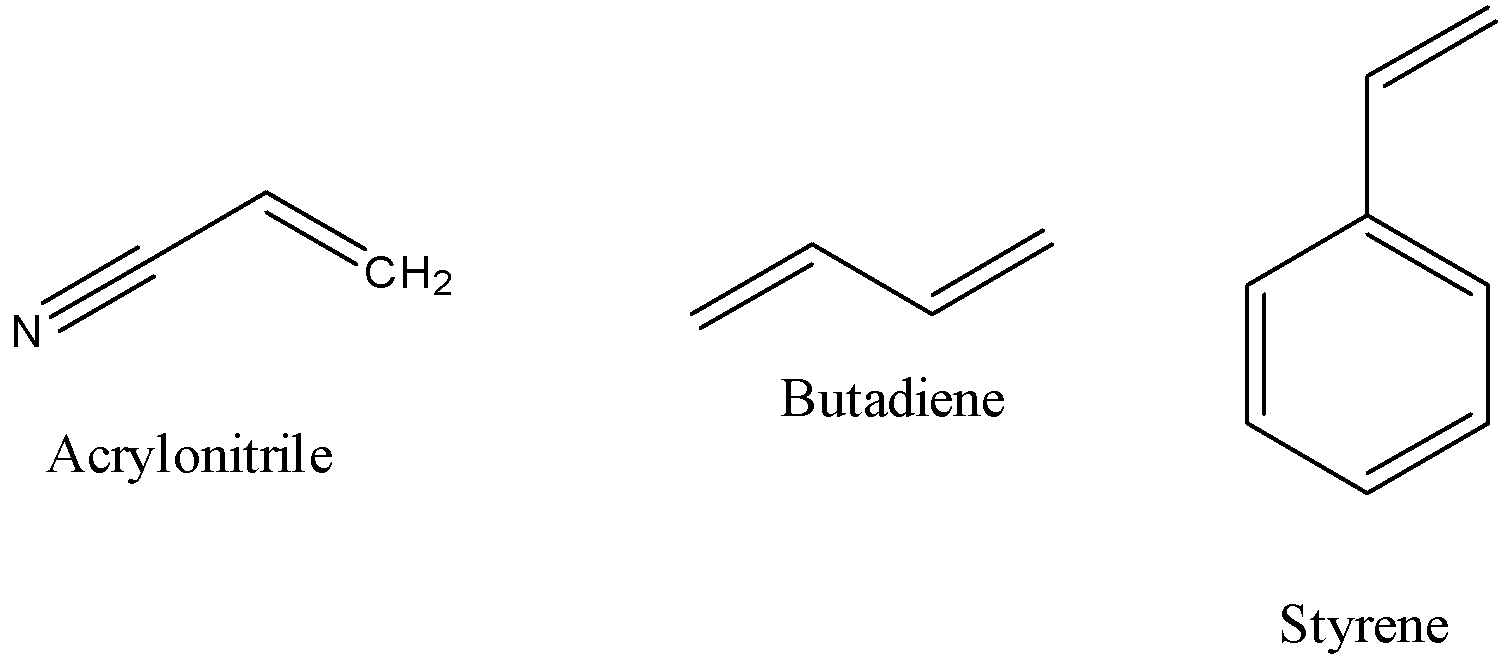
- The polymerization reaction which involves the formation of ABS plastic with the help of three monomers (acrylonitrile, butadiene and styrene) is as follows.
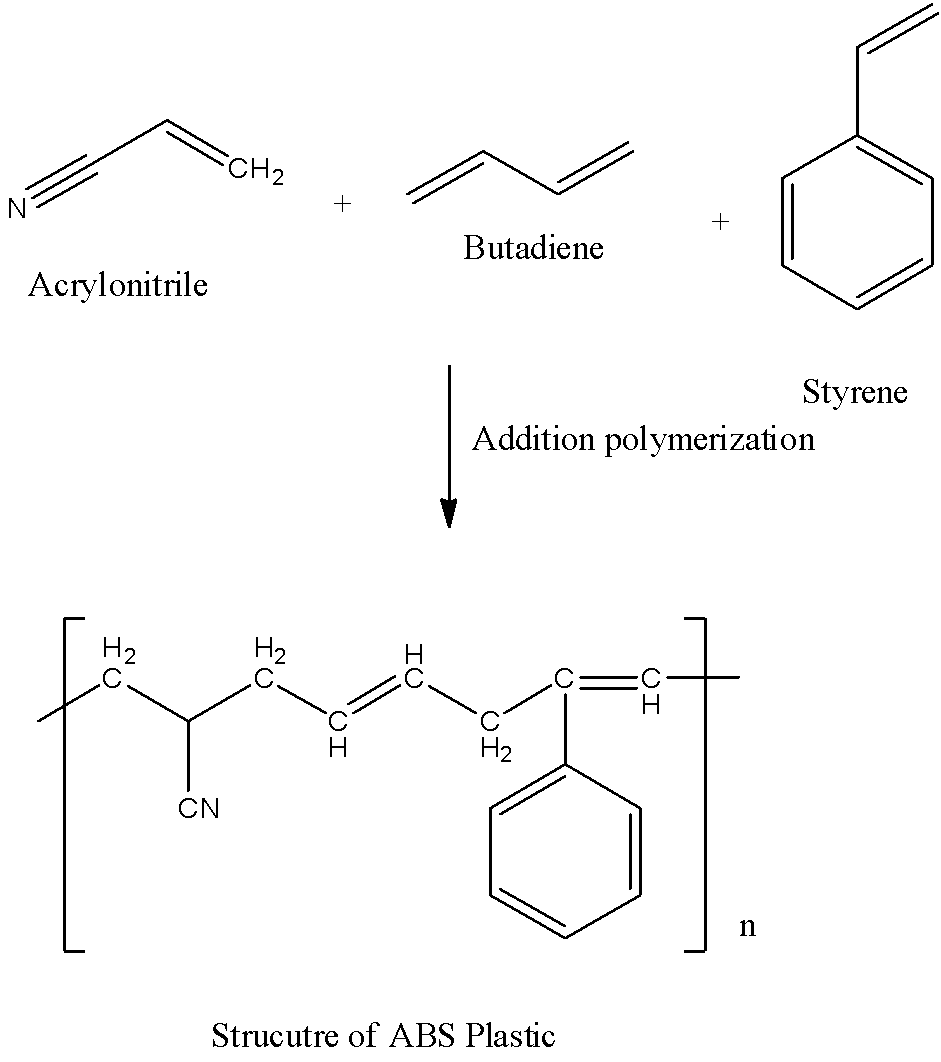
- The acrylonitrile in Abs plastic gives its carbons to form a long chain and also provides thermal stability to ABS plastic.
- The butadiene provides the toughness and strength to the Abs plastic.
- The monomer styrene provides the glossy finish to the ABS plastic.
Note: In the name ABS plastic, A is nothing but acrylonitrile, B is nothing but butadiene and S is nothing but styrene. ABS plastic is highly used in the manufacturing of drain waste vent pies. Few musical instruments like recorders, piano movements and plastic oboes are going to prepare using ABS plastic.
