Question
Question: A plot of volume (V) versus temperature (T) for a gas at constant pressure is a straight line passin...
A plot of volume (V) versus temperature (T) for a gas at constant pressure is a straight line passing through the origin. The plots at different values of pressure are shown in figure.
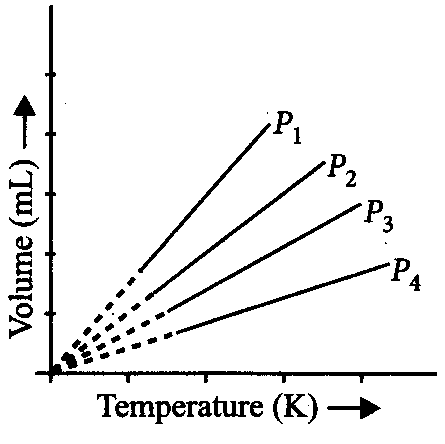
Which of the following order of pressure is correct for this gas?
A

B
P1=P2=P3=P4
C
P1<P2<P3<P4
D
P1<P2=P3<P4
Answer
P1<P2<P3<P4
Explanation
Solution
: PV = constant at given temperature
∴P1 V1=P2 V2=P3 V3=P4 V4
Now, V1>V2>V3>V4 (From the figures)
Hence, P1<P2<P3<P4 (∵P∝ V1)
