Question
Question: A photochemically-induced electrocyclic reaction involves which of a molecule's molecular orbitals? ...
A photochemically-induced electrocyclic reaction involves which of a molecule's molecular orbitals?
A: LUMO
B: LUMO + 1
C: HOMO
D: HOMO - 1
Solution
An electrocyclic reaction involves a pericyclic rearrangement which means that one pi bond can be converted into one sigma bond and vice versa. Electrocyclic reactions can either be photochemically-induced or thermally-induced. Moreover, these reactions can either be ring-opening or ring-closing.
Complete step by step answer:
Electrocyclic reactions are of two types i.e. thermally-induced and photochemically-induced electrocyclic reactions. In thermally-induced electrocyclic reaction, a molecule for e.g. cyclobutene undergoes a conrotation (both ends rotate in similar direction). On the other hand in photochemically-induced electrocyclic reaction (which uses light as a source of energy), light with a certain frequency excites the electrons to a higher energy level resulting into the change in the HOMO i.e. highest occupied molecular orbital of the reaction. It also results into the alteration of the rotation type that the molecules have to make in order to form and dissociate the bonds during the execution of reaction (relative to the thermally-induced electrocyclic reaction). In case of cyclobutene, the method of rotation is disrotation (both ends rotate exactly in opposite directions) not conrotation. These two cases are demonstrated below:
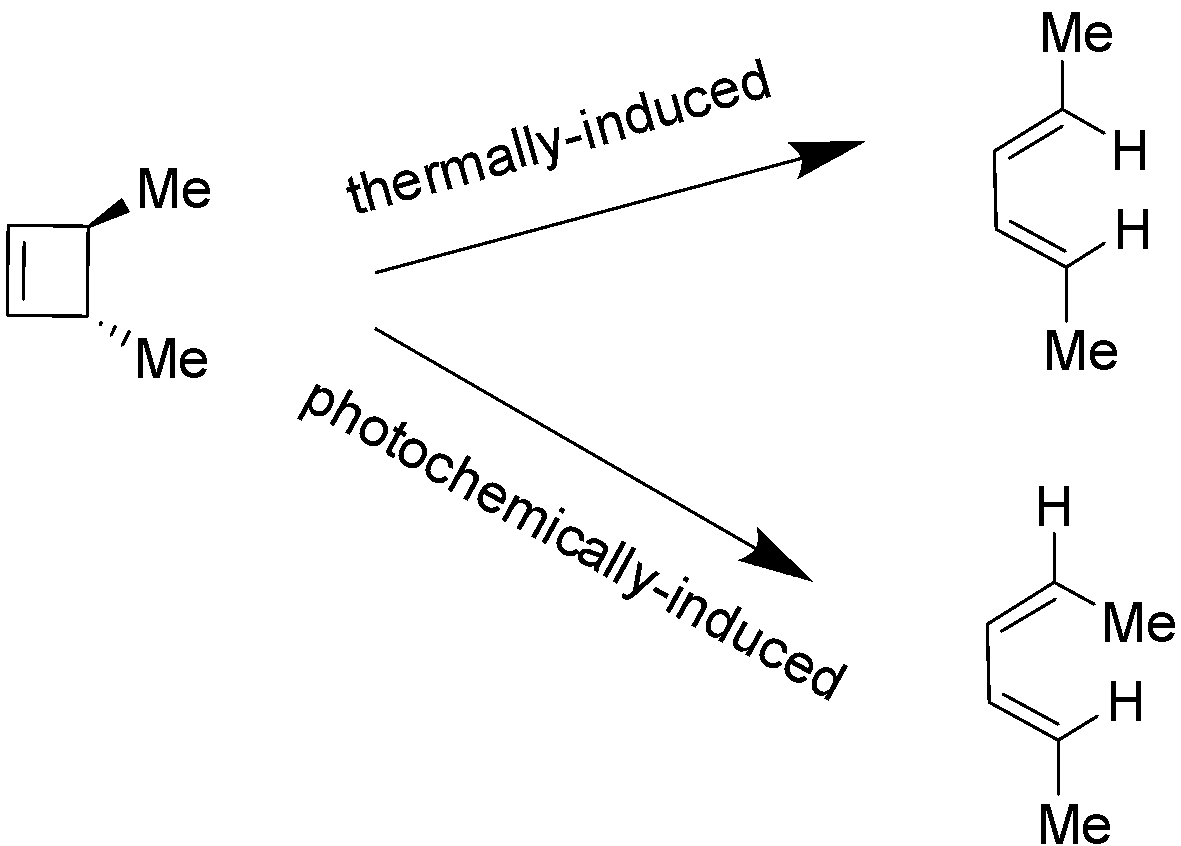
Hence, the correct answer is Option C i.e. A photochemically-induced electrocyclic reaction involves HOMO (highest occupied molecular orbitals).
Note:
If you want to determine whether a specific reaction is either conrotatory or disrotatory, you can accomplish it by identifying the molecular orbitals of all molecules and then following certain rules. You just have to remember few facts/rules to determine either conrotation or disrotation i.e. you should know how many electrons are there in the pi-system and you should know whether the reaction is thermally-induced (i.e. by heat) or photochemically-induced (i.e. by light).
