Question
Question: A particle of mass $m$ moves along a circular orbit in a centro-symmetrical potential field $U(r)=U_...
A particle of mass m moves along a circular orbit in a centro-symmetrical potential field U(r)=U0r2 where U0 is a constant and r is the distance of the particle from the centre of the potential field. Use Bohr quantization condition. En and rn respectively are the energy and radius of the nth state. Then
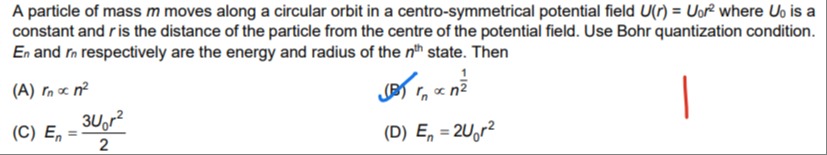
rn∝n2
rn∝n21
En=23U0r2
En=2U0r2
B
Solution
The particle of mass m moves in a centro-symmetrical potential field U(r)=U0r2. The force acting on the particle is F(r)=−drdU=−drd(U0r2)=−2U0r. The magnitude of the force is F(r)=2U0r. This force is attractive and acts towards the center, providing the centripetal force for the circular orbit. For a circular orbit of radius r, the centripetal force is rmv2, where v is the speed of the particle. So, rmv2=2U0r. This gives mv2=2U0r2.
The kinetic energy of the particle is K=21mv2=21(2U0r2)=U0r2. The potential energy is given as U(r)=U0r2. The total energy of the particle in a circular orbit of radius r is E=K+U=U0r2+U0r2=2U0r2. So, for the nth state with radius rn and energy En, the energy is related to the radius by En=2U0rn2.
Now, we apply the Bohr quantization condition for angular momentum, which states that the angular momentum L is quantized: L=mvr=n2πh, where n is a positive integer (n=1,2,3,…) and h is Planck's constant. From the force equation, mv2=2U0r2, we get v2=m2U0r2, so v=rm2U0. Substitute this expression for v into the Bohr quantization condition:
m(rm2U0)r=n2πh
mr2m2U0=n2πh
r2m2m2U0=n2πh
r22mU0=n2πh
Let rn be the radius of the nth state.
rn2=n2π2mU0h
rn=(2π2mU0h)1/2n1/2.
Since 2π2mU0h is a constant, we have rn∝n1/2.
