Question
Question: A non-metal ‘M’ forms \({\text{MC}}{{\text{l}}_{\text{3}}}\), \({{\text{M}}_{\text{2}}}{{\text{O}}_{...
A non-metal ‘M’ forms MCl3, M2O5 and Mg3M2 but does not form MI6. The incorrect statement regarding non-metal ‘M’ is-
A.M can form multiple bond
B.M is of second period element
C.Atomicity of non-metal is 4
D.The range of oxidation number for M is +5 to -3
Solution
In the compounds MCl3, M2O5 and Mg3M2, determine the oxidation number of ‘M’. Also, determine the structures of MCl3, M2O5 and Mg3M2 so that we can determine the bonds formed by the non-metal.
Complete step by step answer:
We are given compounds formed by non-metal ‘M’ which are MCl3, M2O5 and Mg3M2.
In compounds MCl3, we can see that non-metal ‘M’ forms three bonds with three chlorine atoms, in compound M2O5 non-metal ‘M’ forms more than one bond with the five oxygen atoms and in compound Mg3M2 non-metal ‘M’ forms more than one bond with the three magnesium atoms.
We can determine the valency of non-metal ‘M’ by drawing the structures of the compounds MCl3, M2O5 and Mg3M2.
The structures of MCl3, M2O5 and Mg3M2 are as follows:
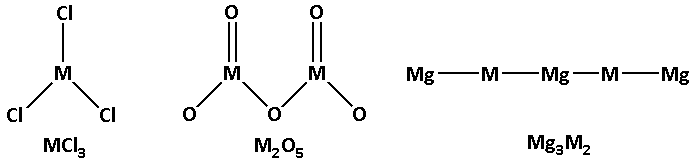
From the structures we can see that the non-metal ‘M’ forms multiple bonds. Thus, the statement ‘M can form multiple bonds’ is correct. Thus, option (A) is not correct.
The compounds formed by the non-metal ‘M’ are MCl3, M2O5 and Mg3M2.
The oxidation state of the non-metal ‘M’ in MCl3 is +3. The oxidation state of the non-metal ‘M’ in M2O5 is +5. And the oxidation state of the non-metal ‘M’ in Mg3M2 is -3
Thus, the statement ‘the range of oxidation numbers for M is +5 to -3’ is correct. Thus, option (D) is correct.
If the non-metal ‘M’ is a second period element then the atomicity of the non-metal ‘M’ is 2.
Thus, the statement ‘M is of second period element’ is correct. Thus, option (B) is not correct.
Thus, the statement ‘atomicity of non-metal is 4’ is incorrect.
Thus, the correct option is option (C).
Note:
The number of atoms that make up or compose its molecule is known as atomicity. The non-metal ‘M’ is a second period element and thus, the atomicity of the non-metal ‘M’ is 2. Thus, the molecule of non-metal is M2.
